Repeated cortico-striatal stimulation generates persistent OCD-like behavior
- PMID: 23744948
- PMCID: PMC3954809
- DOI: 10.1126/science.1234733
Repeated cortico-striatal stimulation generates persistent OCD-like behavior
Abstract
Although cortico-striato-thalamo-cortical (CSTC) circuit dysregulation is correlated with obsessive compulsive disorder (OCD), causation cannot be tested in humans. We used optogenetics in mice to simulate CSTC hyperactivation observed in OCD patients. Whereas acute orbitofrontal cortex (OFC)-ventromedial striatum (VMS) stimulation did not produce repetitive behaviors, repeated hyperactivation over multiple days generated a progressive increase in grooming, a mouse behavior related to OCD. Increased grooming persisted for 2 weeks after stimulation cessation. The grooming increase was temporally coupled with a progressive increase in light-evoked firing of postsynaptic VMS cells. Both increased grooming and evoked firing were reversed by chronic fluoxetine, a first-line OCD treatment. Brief but repeated episodes of abnormal circuit activity may thus set the stage for the development of persistent psychopathology.
Figures

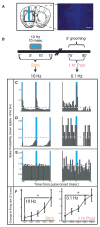
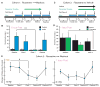
Comment in
-
Neuroscience. Illuminating the neural circuitry of compulsive behaviors.Science. 2013 Jun 7;340(6137):1174-5. doi: 10.1126/science.1239652. Science. 2013. PMID: 23744931 Free PMC article.
-
Psychiatric disorders: repetitive circuits.Nat Rev Neurosci. 2013 Aug;14(8):522. doi: 10.1038/nrn3547. Epub 2013 Jun 26. Nat Rev Neurosci. 2013. PMID: 23801045 No abstract available.
-
A step forward in elucidating the mystery of OCD.Eur Arch Psychiatry Clin Neurosci. 2015 Dec;265(8):735-6. doi: 10.1007/s00406-015-0584-2. Epub 2015 Feb 24. Eur Arch Psychiatry Clin Neurosci. 2015. PMID: 25708456
References
-
- Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020, Global burden of disease and injury series. Vol. 1. Harvard School of Public Health, Harvard Univ Press; Cambridge, MA: 1996.
-
- Kessler RC, et al. Arch Gen Psychiatry. 2005;62:593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

