Flt-1 (vascular endothelial growth factor receptor-1) is essential for the vascular endothelial growth factor-Notch feedback loop during angiogenesis
- PMID: 23744993
- PMCID: PMC4521230
- DOI: 10.1161/ATVBAHA.113.301805
Flt-1 (vascular endothelial growth factor receptor-1) is essential for the vascular endothelial growth factor-Notch feedback loop during angiogenesis
Abstract
Objective: Vascular endothelial growth factor (VEGF) signaling induces Notch signaling during angiogenesis. Flt-1/VEGF receptor-1 negatively modulates VEGF signaling. Therefore, we tested the hypothesis that disrupted Flt-1 regulation of VEGF signaling causes Notch pathway defects that contribute to dysmorphogenesis of Flt-1 mutant vessels.
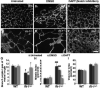
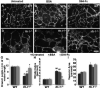
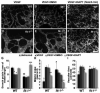
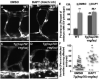
Approach and results: Wild-type and flt-1(-/-) mouse embryonic stem cell-derived vessels were exposed to pharmacological and protein-based Notch inhibitors with and without added VEGF. Vessel morphology, endothelial cell proliferation, and Notch target gene expression levels were assessed. Similar pathway manipulations were performed in developing vessels of zebrafish embryos. Notch inhibition reduced flt-1(-/-) embryonic stem cell-derived vessel branching dysmorphogenesis and endothelial hyperproliferation, and rescue of flt-1(-/-) vessels was accompanied by a reduction in elevated Notch targets. Surprisingly, wild-type vessel morphogenesis and proliferation were unaffected by Notch suppression, Notch targets in wild-type endothelium were unchanged, and Notch suppression perturbed zebrafish intersegmental vessels but not caudal vein plexuses. In contrast, exogenous VEGF caused wild-type embryonic stem cell-derived vessel and zebrafish intersegmental vessel dysmorphogenesis that was rescued by Notch blockade.
Conclusions: Elevated Notch signaling downstream of perturbed VEGF signaling contributes to aberrant flt-1(-/-) blood vessel formation. Notch signaling may be dispensable for blood vessel formation when VEGF signaling is below a critical threshold.
Keywords: Flt-1 protein, mouse; Notch receptors; angiogenesis; embryonic stem cells; vascular endothelial growth factor A; zebrafish.
Figures


References
-
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. - PubMed
-
- Dorrell MI, Aguilar E, Friedlander M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Invest Ophthalmol Vis Sci. 2002;43:3500–3510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

