Ablation of the X-linked retinitis pigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration
- PMID: 23745007
- PMCID: PMC3700388
- DOI: 10.1167/iovs.13-12140
Ablation of the X-linked retinitis pigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration
Abstract
Purpose: Mutations in the RP2 gene are associated with 10% to 15% of X-linked retinitis pigmentosa (XLRP), a debilitating disorder characterized by the degeneration of retinal rod and cone photoreceptors. The molecular mechanism of pathogenesis of photoreceptor degeneration in XLRP-RP2 has not been elucidated, and no treatment is currently available. This study was undertaken to investigate the pathogenesis of RP2-associated retinal degeneration.
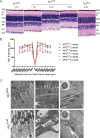
Methods: We introduced loxP sites that flank exon 2, a mutational hotspot in XLRP-RP2, in the mouse Rp2 gene. We then produced Rp2-null allele using transgenic mice that expressed Cre-recombinase under control of the ubiquitous CAG promoter. Electroretinography (ERG), histology, light microscopy, transmission electron microscopy, and immunofluorescence microscopy were performed to ascertain the effect of ablation of Rp2 on photoreceptor development, function, and protein trafficking.
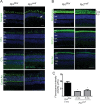
Results: Although no gross abnormalities were detected in the Rp2(null) mice, photopic (cone) and scotopic (rod) function as measured by ERG showed a gradual decline starting as early as 1 month of age. We also detected slow progressive degeneration of the photoreceptor membrane discs in the mutant retina. These defects were associated with mislocalization of cone opsins to the nuclear and synaptic layers and reduced rhodopsin content in the outer segment of mutant retina prior to the onset of photoreceptor degeneration.
Conclusions: Our studies suggest that RP2 contributes to the maintenance of photoreceptor function and that cone opsin mislocalization represents an early step in XLRP caused by RP2 mutations. The Rp2(null) mice should serve as a useful preclinical model for testing gene- and cell-based therapies.
Keywords: Rp2; photoreceptor; retina.
Figures



References
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010; 11: 273–284 - PubMed
-
- Nathans J, Piantanida TP, Eddy RL, Shows TB, Hogness DS. Molecular genetics of inherited variation in human color vision. Science. 1986; 232: 203–210 - PubMed
-
- Neitz M, Neitz J, Jacobs GH. Spectral tuning of pigments underlying red-green color vision. Science. 1991; 252: 971–974 - PubMed
-
- Deretic D. Post-Golgi trafficking of rhodopsin in retinal photoreceptors. Eye. 1998; 12: 526–530 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

