Stage and size using magnetic resonance imaging and endosonography in neoadjuvantly-treated rectal cancer
- PMID: 23745028
- PMCID: PMC3671078
- DOI: 10.3748/wjg.v19.i21.3263
Stage and size using magnetic resonance imaging and endosonography in neoadjuvantly-treated rectal cancer
Abstract
Aim: To assess the stage and size of rectal tumours using 1.5 Tesla (1.5T) magnetic resonance imaging (MRI) and three-dimensional (3D) endosonography (ERUS).
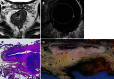
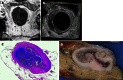
Methods: In this study, patients were recruited in a phase I/II trial of neoadjuvant chemotherapy for biopsy-proven rectal cancer planned for surgical resection with or without preoperative radiotherapy. The feasibility and accuracy of 1.5T MRI and 3D ERUS were compared with the histopathology of the fixed surgical specimen (pathology) to determine the stage and size of the rectal cancer before and after neoadjuvant chemotherapy. A Philips Intera 1.5T with a cardiac 5-channel synergy surface coil was used for the MRI, and a B-K Medical Falcon 2101 EXL 3D-Probe was used at 13 MHz for the ERUS. Our hypothesis was that the staging accuracy would be the same when using MRI, ERUS and a combination of MRI and ERUS. For the combination, MRI was chosen for the assessment of the lymph nodes, and ERUS was chosen for the assessment of perirectal tissue penetration. The stage was dichotomised into stage I and stage II or greater. The size was measured as the supero-inferior length and the maximal transaxial area of the tumour.
Results: The staging feasibility was 37 of 37 for the MRI and 29 of 36 for the ERUS, with stenosis as a limiting factor. Complete sets of investigations were available in 18 patients for size and 23 patients for stage. The stage accuracy by MRI, ERUS and the combination of MRI and ERUS was 0.65, 0.70 and 0.74, respectively, before chemotherapy and 0.65, 0.78 and 0.83, respectively, after chemotherapy. The improvement of the post-chemotherapy staging using the combination of MRI and ERUS compared with the staging using MRI alone was significant (P = 0.046). The post-chemotherapy understaging frequency by MRI, ERUS and the combination of MRI and ERUS was 0.18, 0.14 and 0.045, respectively, and these differences were non-significant. The measurements of the supero-inferior length by ERUS compared with MRI were within 1.96 standard deviations of the difference between the methods (18 mm) for tumours smaller than 50 mm. The agreement with pathology was within 1.96 standard deviations of the difference between imaging and pathology for all tumours with MRI (15 mm) and for tumours that did not exceed 50 mm with ERUS (22 mm). Tumours exceeding 50 mm in length could not be reliably measured by ERUS due to the limit in the length of each recording.
Conclusion: MRI is preferable to use when assessing the size of large or stenotic rectal tumours. However, staging accuracy is improved by combining MRI with ERUS.
Keywords: Endosonography; Magnetic resonance imaging; Neoadjuvant treatment; Predictive value of tests; Rectal cancer.
Figures

References
-
- Blomqvist L, Glimelius B. The ‘good’, the ‘bad’, and the ‘ugly’ rectal cancers. Acta Oncol. 2008;47:5–8. - PubMed
-
- Derwinger K, Kodeda K, Swartling T, Kälebo P, Carlsson G, Gustavsson B. A phase I/II study of neoadjuvant chemotherapy with Pemetrexed (Alimta) in rectal cancer. Eur J Surg Oncol. 2011;37:583–588. - PubMed
-
- Smith N, Brown G. Preoperative staging of rectal cancer. Acta Oncol. 2008;47:20–31. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

