A fluorogenic TMP-tag for high signal-to-background intracellular live cell imaging
- PMID: 23745575
- PMCID: PMC3800097
- DOI: 10.1021/cb300657r
A fluorogenic TMP-tag for high signal-to-background intracellular live cell imaging
Abstract
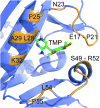
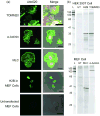
Developed to complement the use of fluorescent proteins in live cell imaging, chemical tags enjoy the benefit of modular incorporation of organic fluorophores, opening the possibility of high photon output and special photophysical properties. However, the theoretical challenge in using chemical tags as opposed to fluorescent proteins for high-resolution imaging is background noise from unbound and/or nonspecifically bound ligand-fluorophore. We envisioned we could overcome this limit by engineering fluorogenic trimethoprim-based chemical tags (TMP-tags) in which the fluorophore is quenched until binding with E. coli dihydrofolate reductase (eDHFR)-tagged protein displaces the quencher. Thus, we began by building a nonfluorogenic, covalent TMP-tag based on a proximity-induced reaction known to achieve rapid and specific labeling both in vitro and inside of living cells. Here we take the final step and render the covalent TMP-tag fluorogenic. In brief, we designed a trimeric TMP-fluorophore-quencher molecule (TMP-Q-Atto520) with the quencher attached to a leaving group that, upon TMP binding to eDHFR, would be cleaved by a cysteine residue (Cys) installed just outside the binding pocket of eDHFR. We present the in vitro experiments showing that the eDHFR:L28C nucleophile cleaves the TMP-Q-Atto520 rapidly and efficiently, resulting in covalent labeling and remarkable fluorescence enhancement. Most significantly, while only our initial design, TMP-Q-Atto520 achieved the demanding goal of not only labeling highly abundant, localized intracellular proteins but also less abundant, more dynamic cytoplasmic proteins. These results suggest that the fluorogenic TMP-tag can significantly impact high-resolution live cell imaging and further establish the potential of proximity-induced reactivity and organic chemistry more broadly as part of the growing toolbox for synthetic biology and cell engineering.
Figures





References
-
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. - PubMed
-
- Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. - PubMed
-
- Ormo M, Cubitt AB, Kallio K, Gross LA, Tsien RY, Remington SJ. Crystal structure of the Aequorea victoria green fluorescent protein. Science. 1996;273:1392–1395. - PubMed
-
- Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120:4247–4260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

