High variability in baseline urinary free cortisol values in patients with Cushing's disease
- PMID: 23746264
- PMCID: PMC4231220
- DOI: 10.1111/cen.12259
High variability in baseline urinary free cortisol values in patients with Cushing's disease
Abstract
Objective: Twenty-four-hour urinary free cortisol (UFC) sampling is commonly used to evaluate Cushing's syndrome. Because there are few data on UFC variability in patients with active Cushing's disease, we analysed baseline UFC in a large patient cohort with moderate-to-severe Cushing's disease and assessed whether variability correlates with hypercortisolism severity. These data will help clinicians establish the minimum number of UFC samples required to obtain reliable data.
Design: Observational study (enrolment phase of Phase III study).
Methods: Patients (n = 152) with persistent/recurrent or de novo Cushing's disease and mean UFC (mUFC) ≥1·5×ULN (normal: 30-145 nmol/24 h) were included. Mean UFC level was calculated from four 24-h urine samples collected over 2 weeks.
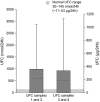
Results: Over 600 24-h UFC samples were analysed. The mUFC levels of samples 1 and 2 and samples 3 and 4 were 1000 nmol/24 h (SD 1872) and 940 nmol/24 h (SD 2148), respectively; intrapatient coefficient of variation (CV) was 38% for mUFC. The intrapatient CV using all four samples was 52% (95% CI: 48-56). The intrapatient CV was 51% (95% CI: 44-58) for samples 1 and 2, 49% (95% CI: 43-56) for samples 3 and 4 and 54% (95% CI: 49-59) for samples 1, 2 and 3. Variability in mUFC increased as UFC levels increased. There were no correlations between UFC and clinical features of hypercortisolism.
Conclusions: There is intrapatient variability of approximately 50% in 24-h UFC measurements, which is relevant to targets set to estimate any treatment effect. Analysing more than two 24-h collection periods in individual patients does not result in a relevant decrease in variability. Interestingly, UFC levels did not correlate with hypercortisolism severity.
Trial registration: ClinicalTrials.gov NCT00434148.
© 2013 The Authors. Clinical Endocrinology published by John Wiley & Sons Ltd.
Figures



References
-
- Bertagna X, Guignat L, Groussin L, et al. Cushing’s disease. Best Practice and Research Clinical Endocrinology and Metabolism. 2009;23:607–623. - PubMed
-
- Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. The Lancet. 2006;367:1605–1617. - PubMed
-
- Clayton RN, Raskauskiene D, Reulen RC, et al. Mortality and morbidity in Cushing’s disease over 50 years in Stoke-on-Trent, UK: audit and meta-analysis of literature. Journal of Clinical Endocrinology and Metabolism. 2011;96:632–642. - PubMed
-
- Webb SM, Badia X, Barahona MJ, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. European Journal of Endocrinology. 2008;158:623–630. - PubMed
-
- Etxabe J. Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clinical Endocrinology. 1994;40:479–484. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

