Correlation of pre-existing vascular pathology with arteriovenous graft outcomes in hemodialysis patients
- PMID: 23746379
- PMCID: PMC3778052
- DOI: 10.1053/j.ajkd.2013.03.040
Correlation of pre-existing vascular pathology with arteriovenous graft outcomes in hemodialysis patients
Abstract
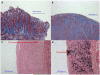
Background: Arteriovenous grafts (AVGs) are prone to neointimal hyperplasia leading to AVG failure. We hypothesized that pre-existing pathologic abnormalities of the vessels used to create AVGs (including venous intimal hyperplasia, arterial intimal hyperplasia, arterial medial fibrosis, and arterial calcification) are associated with inferior AVG survival.
Study design: Prospective observational study.
Setting & participants: Patients with chronic kidney disease undergoing placement of a new AVG at a large medical center who had vascular specimens obtained at the time of surgery (n = 76).
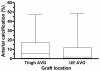
Predictor: Maximal intimal thickness of the arterial and venous intima, arterial medial fibrosis, and arterial medial calcification.
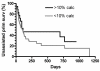
Outcome & measurements: Unassisted primary AVG survival (time to first intervention) and frequency of AVG interventions.
Results: 55 patients (72%) underwent interventions and 148 graft interventions occurred during 89.9 years of follow-up (1.65 interventions per graft-year). Unassisted primary AVG survival was not associated significantly with arterial intimal thickness (HR, 0.72; 95% CI, 0.40-1.27; P = 0.3), venous intimal thickness (HR, 0.64; 95% CI, 0.37-1.10; P = 0.1), severe arterial medial fibrosis (HR, 0.58; 95% CI, 0.32-1.06; P = 0.6), or severe arterial calcification (HR, 0.68; 95% CI, 0.37-1.31; P = 0.3). The frequency of AVG interventions per year was associated inversely with arterial intimal thickness (relative risk [RR], 1.99; 95% CI, 1.16-3.42; P < 0.001 for thickness <10 vs. >25 μm), venous intimal thickness (RR, 2.11; 95% CI, 1.39-3.20; P < 0.001 for thickness <5 vs. >10 μm), arterial medial fibrosis (RR, 3.17; 95% CI, 1.96-5.13; P < 0.001 for fibrosis <70% vs. ≥70%), and arterial calcification (RR, 2.12; 95% CI, 1.31-3.43; P = 0.001 for <10% vs. ≥10% calcification).
Limitations: Single-center study. Study may be underpowered to demonstrate differences in unassisted primary AVG survival.
Conclusions: Pre-existing vascular pathologic abnormalities in patients with chronic kidney disease may not be associated significantly with unassisted primary AVG survival. However, vascular intimal hyperplasia, arterial medial fibrosis, and arterial calcification may be associated with a decreased frequency of AVG interventions.
Keywords: Arteriovenous graft; intimal hyperplasia; medial fibrosis; vascular calcification.
Copyright © 2013 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Allon M. Current management of vascular access. Clin J Am Soc Nephrol. 2007;2:786–800. - PubMed
-
- Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–34. - PubMed
-
- Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: A cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–27. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

