Substrate-selective repair and restart of replication forks by DNA translocases
- PMID: 23746452
- PMCID: PMC3700663
- DOI: 10.1016/j.celrep.2013.05.002
Substrate-selective repair and restart of replication forks by DNA translocases
Abstract
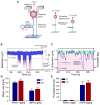
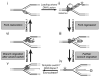
Stalled replication forks are sources of genetic instability. Multiple fork-remodeling enzymes are recruited to stalled forks, but how they work to promote fork restart is poorly understood. By combining ensemble biochemical assays and single-molecule studies with magnetic tweezers, we show that SMARCAL1 branch migration and DNA-annealing activities are directed by the single-stranded DNA-binding protein RPA to selectively regress stalled replication forks caused by blockage to the leading-strand polymerase and to restore normal replication forks with a lagging-strand gap. We unveil the molecular mechanisms by which RPA enforces SMARCAL1 substrate preference. E. coli RecG acts similarly to SMARCAL1 in the presence of E. coli SSB, whereas the highly related human protein ZRANB3 has different substrate preferences. Our findings identify the important substrates of SMARCAL1 in fork repair, suggest that RecG and SMARCAL1 are functional orthologs, and provide a comprehensive model of fork repair by these DNA translocases.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




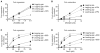
References
-
- Baradaran-Heravi A, Raams A, Lubieniecka J, Cho KS, DeHaai KA, Basiratnia M, Mari PO, Xue Y, Rauth M, Olney AH, et al. SMARCAL1 deficiency predisposes to non-Hodgkin lymphoma and hypersensitivity to genotoxic agents in vivo. American journal of medical genetics Part A. 2012;158A:2204–2213. - PMC - PubMed
-
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol. 2010;11:208–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

