Structural flexibility of CaV1.2 and CaV2.2 I-II proximal linker fragments in solution
- PMID: 23746511
- PMCID: PMC3672870
- DOI: 10.1016/j.bpj.2013.04.034
Structural flexibility of CaV1.2 and CaV2.2 I-II proximal linker fragments in solution
Abstract
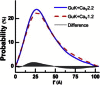
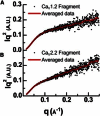
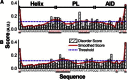
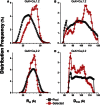
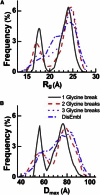
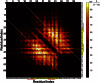
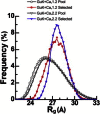
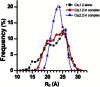
Voltage-dependent calcium channels (CaV) enable the inward flow of calcium currents for a wide range of cells. CaV1 and CaV2 subtype α1 subunits form the conducting pore using four repeated membrane domains connected by intracellular linkers. The domain I-II linker connects to the membrane gate (IS6), forming an α-helix, and is bound to the CaVβ subunit. Previous studies indicated that this region may or may not form a continuous helix depending on the CaV subtype, thereby modulating channel activation and inactivation properties. Here, we used small-angle x-ray scattering and ensemble modeling analysis to investigate the solution structure of these linkers, extending from the membrane domain and including the CaVβ-binding site, called the proximal linker (PL). The results demonstrate that the CaV1.2 PL is more flexible than the CaV2.2 PL, the flexibility is intrinsic and not dependent on CaVβ binding, and the flexibility can be most easily explained by the presence of conserved glycines. Our analysis also provides a robust example of investigating protein domains in which flexibility plays an essential role.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Jones S.W. Overview of voltage-dependent calcium channels. J. Bioenerg. Biomembr. 1998;30:299–312. - PubMed
-
- Chen Y.H., Li M.H., Yang J. Structural basis of the α1-β subunit interaction of voltage-gated Ca2+ channels. Nature. 2004;429:675–680. - PubMed
-
- Opatowsky Y., Chen C.C., Hirsch J.A. Structural analysis of the voltage-dependent calcium channel β subunit functional core and its complex with the α1 interaction domain. Neuron. 2004;42:387–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

