Effector-like CD8⁺ T cells in the memory population mediate potent protective immunity
- PMID: 23746652
- PMCID: PMC3703254
- DOI: 10.1016/j.immuni.2013.05.009
Effector-like CD8⁺ T cells in the memory population mediate potent protective immunity
Abstract
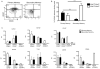
The CD8⁺ memory T cell population is heterogeneous, and it is unclear which subset(s) optimally mediate the central goal of the immune system-protection against infection. Here we investigate the protective capacities of CD8⁺ T cell subsets present at the memory stage of the immune response. We show that a population of CD8⁺ T cells bearing markers associated with effector cells (KLRG1(hi), CD27(lo), T-bet(hi), Eomes(lo)) persisted to the memory phase and provided optimal control of Listeria monocytogenes and vaccinia virus, despite weak recall proliferative responses. After antigen-specific boosting, this population formed the predominant secondary memory subset and maintained superior pathogen control. The effector-like memory subset displayed a distinct pattern of tissue distribution and localization within the spleen, and their enhanced capacity to eliminate Listeria involved specialized utilization of cytolysis. Together, these data suggest that long-lived effector CD8⁺ T cells are optimal for protective immunity against certain pathogens.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


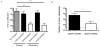


Comment in
-
Instant recall: a key role for effector-phenotype CD8⁺ memory T cells in immune protection.Immunity. 2013 Jun 27;38(6):1090-1. doi: 10.1016/j.immuni.2013.06.007. Immunity. 2013. PMID: 23809159
References
-
- Baars PA, Sierro S, Arens R, Tesselaar K, Hooibrink B, Klenerman P, van Lier RA. Properties of murine (CD8+)CD27- T cells. Eur J Immunol. 2005;35:3131–3141. - PubMed
-
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005a;175:4686–4696. - PubMed
-
- Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005b;175:4677–4685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

