Cross-regulation of T regulatory-cell response after coxsackievirus B3 infection by NKT and γδ T cells in the mouse
- PMID: 23746656
- PMCID: PMC3730787
- DOI: 10.1016/j.ajpath.2013.04.015
Cross-regulation of T regulatory-cell response after coxsackievirus B3 infection by NKT and γδ T cells in the mouse
Abstract
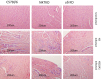
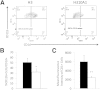
Coxsackievirus B3 (CVB3) variants H3 and H310A1 differ by a single nonconserved amino acid in the VP2 capsid region. C57Bl/6 mice infected with the H3 virus develop myocarditis correlating with activation of T cells expressing the Vγ4 T cell receptor chain. Infecting mice with H310A1 activates natural killer T (NKT; mCD1d-tetramer(+) TCRβ(+)) cells, but not Vγ4 T cells, and fails to induce myocarditis. H310A1 infection preferentially activates M2 alternatively activated macrophage and CD4(+)FoxP3 (T regulatory) cells, whereas CD4(+)Th1 (IFN-γ(+)) cells are suppressed. By contrast, H3 virus infection activates M1 proinflammatory and CD4(+)Th1 cells, but not T regulatory cells. The M1 macrophage show significantly increased CD1d expression compared to M2 macrophage. The ability of NKT cells to suppress myocarditis was shown by adoptive transfer of purified NKT cells into H3-infected NKT knockout (Jα18 knockout) mice, which inhibited cardiac inflammation and increased T regulatory cell response. Cardiac virus titers were equivalent in all mouse strains indicating that neither Vγ4 nor NKT cells participate in control of virus infection. These data show that NKT and Vγ4 cells cross-regulate T regulatory cell responses during CVB3 infections and are the primary factor determining viral pathogenesis in this mouse model.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis.J Virol. 2001 Jul;75(13):5860-9. doi: 10.1128/JVI.75.13.5860-5869.2001. J Virol. 2001. PMID: 11390587 Free PMC article.
-
Slam haplotype 2 promotes NKT but suppresses Vγ4+ T-cell activation in coxsackievirus B3 infection leading to increased liver damage but reduced myocarditis.Am J Pathol. 2013 Feb;182(2):401-9. doi: 10.1016/j.ajpath.2012.10.019. Epub 2012 Nov 27. Am J Pathol. 2013. PMID: 23195432 Free PMC article.
-
Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells.J Virol. 2002 Nov;76(21):10785-90. doi: 10.1128/jvi.76.21.10785-10790.2002. J Virol. 2002. PMID: 12368321 Free PMC article.
-
Cross-talk between cd1d-restricted nkt cells and γδ cells in t regulatory cell response.Virol J. 2011 Jan 21;8:32. doi: 10.1186/1743-422X-8-32. Virol J. 2011. PMID: 21255407 Free PMC article. Review.
-
Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells.Microbes Infect. 2005 Mar;7(3):537-43. doi: 10.1016/j.micinf.2004.12.011. Epub 2005 Feb 5. Microbes Infect. 2005. PMID: 15777711 Review.
Cited by
-
γδ T Cells and dendritic cells in refractory Lyme arthritis.J Leukoc Biol. 2015 Apr;97(4):653-63. doi: 10.1189/jlb.2RU0714-343RR. Epub 2015 Jan 20. J Leukoc Biol. 2015. PMID: 25605869 Free PMC article. Review.
-
Learning from myocarditis: mimicry, chaos and black holes.F1000Prime Rep. 2014 May 6;6:25. doi: 10.12703/P6-25. eCollection 2014. F1000Prime Rep. 2014. PMID: 24904749 Free PMC article. Review.
-
Age-Associated Changes in Estrogen Receptor Ratios Correlate with Increased Female Susceptibility to Coxsackievirus B3-Induced Myocarditis.Front Immunol. 2017 Nov 16;8:1585. doi: 10.3389/fimmu.2017.01585. eCollection 2017. Front Immunol. 2017. PMID: 29201031 Free PMC article. Review.
-
T-cell immunity in myocardial inflammation: pathogenic role and therapeutic manipulation.Br J Pharmacol. 2017 Nov;174(22):3914-3925. doi: 10.1111/bph.13613. Epub 2016 Oct 4. Br J Pharmacol. 2017. PMID: 27590129 Free PMC article. Review.
-
γδ T Cells Mediate a Requisite Portion of a Wound Healing Response Triggered by Cutaneous Poxvirus Infection.Viruses. 2024 Mar 10;16(3):425. doi: 10.3390/v16030425. Viruses. 2024. PMID: 38543790 Free PMC article.
References
-
- Bowles N.E., Ni J., Kearney D.L., Pauschinger M., Schultheiss H.P., McCarthy R., Hare J., Bricker J.T., Bowles K.R., Towbin J.A. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. - PubMed
-
- Freeman G., Colston J., Zabalgoitia M., Chandrasekar B. Contractile depression and expression of proinflammatory cytokines and iNOS in viral myocarditis. Am J Physiol. 1998;274:H249–H258. - PubMed
-
- Maisch B., Bauer E., Cirsi M., Kocksiek K. Cytolytic cross-reactive antibodies directed against the cardiac membrane and viral proteins in coxsackievirus B3 and B4 myocarditis. Circulation. 1993;87(Suppl IV):49–65. - PubMed
-
- Fairweather D., Kaya Z., Shellam G.R., Lawson C.M., Rose N.R. From infection to autoimmunity. J Autoimmun. 2001;16:175–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

