Regulation of organismal proteostasis by transcellular chaperone signaling
- PMID: 23746847
- PMCID: PMC3955170
- DOI: 10.1016/j.cell.2013.05.015
Regulation of organismal proteostasis by transcellular chaperone signaling
Abstract
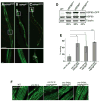
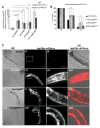
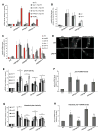
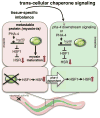
A major challenge for metazoans is to ensure that different tissues, each expressing distinctive proteomes, are nevertheless well protected at an organismal level from proteotoxic stress. We show that expression of endogenous metastable proteins in muscle cells, which rely on chaperones for proper folding, induces a systemic stress response throughout multiple tissues of C. elegans. Suppression of misfolding in muscle cells can be achieved not only by enhanced expression of HSP90 in muscle cells but as effectively by elevated expression of HSP90 in intestine or neuronal cells. This cell-nonautonomous control of HSP90 expression relies upon transcriptional feedback between somatic tissues that is regulated by the FoxA transcription factor PHA-4. This transcellular chaperone signaling response maintains organismal proteostasis when challenged by a local tissue imbalance in folding and provides the basis for organismal stress-sensing surveillance.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Protein metabolism: Proteostasis goes global.Nat Rev Mol Cell Biol. 2013 Aug;14(8):461. doi: 10.1038/nrm3626. Epub 2013 Jul 10. Nat Rev Mol Cell Biol. 2013. PMID: 23839579 No abstract available.
References
-
- Altun-Gultekin ZaHDH. Coelomocyte System. WormAtlas 2009
-
- Barral JM, Hutagalung AH, Brinker A, Hartl FU, Epstein HF. Role of the myosin assembly protein UNC-45 as a molecular chaperone for myosin. Science. 2002;295:669–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

