The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling
- PMID: 23747014
- PMCID: PMC3775267
- DOI: 10.1016/j.molcel.2013.05.003
The proto-oncometabolite fumarate binds glutathione to amplify ROS-dependent signaling
Erratum in
- Mol Cell. 2013 Jul 25;51(2):273
Abstract
The tricarboxylic acid cycle enzyme fumarate hydratase (FH) has been identified as a tumor suppressor in a subset of human renal cell carcinomas. Human FH-deficient cancer cells display high fumarate concentration and ROS levels along with activation of HIF-1. The underlying mechanisms by which FH loss increases ROS and HIF-1 are not fully understood. Here, we report that glutamine-dependent oxidative citric acid cycle metabolism is required to generate fumarate and increase ROS and HIF-1 levels. Accumulated fumarate directly bonds the antioxidant glutathione in vitro and in vivo to produce the metabolite succinated glutathione (GSF). GSF acts as an alternative substrate to glutathione reductase to decrease NADPH levels and enhance mitochondrial ROS and HIF-1 activation. Increased ROS also correlates with hypermethylation of histones in these cells. Thus, fumarate serves as a proto-oncometabolite by binding to glutathione which results in the accumulation of ROS.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


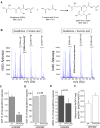



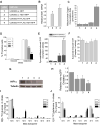
Comment in
-
Mitochondrial metabolism in TCA cycle mutant cancer cells.Cell Cycle. 2014;13(3):347-8. doi: 10.4161/cc.27513. Epub 2013 Dec 13. Cell Cycle. 2014. PMID: 24335489 Free PMC article. No abstract available.
References
-
- Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp Gordon W, Wolhuter K, et al. Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer Cell. 2011;20:524–537. - PMC - PubMed
-
- Alderson NL, Wang Y, Blatnik M, Frizzell N, Walla MD, Lyons TJ, Alt N, Carson JA, Nagai R, Thorpe SR, et al. S-(2-Succinyl)cysteine: A novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. - PubMed
-
- Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, Howarth K, O’Flaherty L, Roberts I, Turner G, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. - PubMed
-
- Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

