Addiction as a stress surfeit disorder
- PMID: 23747571
- PMCID: PMC3830720
- DOI: 10.1016/j.neuropharm.2013.05.024
Addiction as a stress surfeit disorder
Abstract
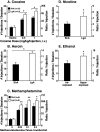
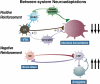
Drug addiction has been conceptualized as a chronically relapsing disorder of compulsive drug seeking and taking that progresses through three stages: binge/intoxication, withdrawal/negative affect, and preoccupation/anticipation. Drug addiction impacts multiple motivational mechanisms and can be conceptualized as a disorder that progresses from positive reinforcement (binge/intoxication stage) to negative reinforcement (withdrawal/negative affect stage). The construct of negative reinforcement is defined as drug taking that alleviates a negative emotional state. Our hypothesis is that the negative emotional state that drives such negative reinforcement is derived from dysregulation of key neurochemical elements involved in the brain stress systems within the frontal cortex, ventral striatum, and extended amygdala. Specific neurochemical elements in these structures include not only recruitment of the classic stress axis mediated by corticotropin-releasing factor (CRF) in the extended amygdala as previously hypothesized but also recruitment of dynorphin-κ opioid aversive systems in the ventral striatum and extended amygdala. Additionally, we hypothesized that these brain stress systems may be engaged in the frontal cortex early in the addiction process. Excessive drug taking engages activation of CRF not only in the extended amygdala, accompanied by anxiety-like states, but also in the medial prefrontal cortex, accompanied by deficits in executive function that may facilitate the transition to compulsive-like responding. Excessive activation of the nucleus accumbens via the release of mesocorticolimbic dopamine or activation of opioid receptors has long been hypothesized to subsequently activate the dynorphin-κ opioid system, which in turn can decrease dopaminergic activity in the mesocorticolimbic dopamine system. Blockade of the κ opioid system can also block anxiety-like and reward deficits associated with withdrawal from drugs of abuse and block the development of compulsive-like responding during extended access to drugs of abuse, suggesting another powerful brain stress/anti-reward system that contributes to compulsive drug seeking. Thus, brain stress response systems are hypothesized to be activated by acute excessive drug intake, to be sensitized during repeated withdrawal, to persist into protracted abstinence, and to contribute to the development and persistence of addiction. The recruitment of anti-reward systems provides a powerful neurochemical basis for the negative emotional states that are responsible for the dark side of addiction. This article is part of a Special Issue entitled 'NIDA 40th Anniversary Issue'.
Keywords: Abstinence or withdrawal; Compulsive; Corticotropin-releasing factor; Dynorphin; Extended amygdala; Impulsive; Opponent process; Prefrontal cortex; Reward; Sensitization.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



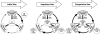
References
-
- Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–571. - PubMed
-
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat. Neurosci. 2002;5:625–626. - PubMed
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. - PubMed
-
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. - PubMed
-
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AA006420/AA/NIAAA NIH HHS/United States
- DK26741/DK/NIDDK NIH HHS/United States
- DA004043/DA/NIDA NIH HHS/United States
- AA020608/AA/NIAAA NIH HHS/United States
- R01 DA023597/DA/NIDA NIH HHS/United States
- P01 DK026741/DK/NIDDK NIH HHS/United States
- DA010072/DA/NIDA NIH HHS/United States
- P60 AA006420/AA/NIAAA NIH HHS/United States
- R01 AA020608/AA/NIAAA NIH HHS/United States
- R01 DA004398/DA/NIDA NIH HHS/United States
- R37 AA008459/AA/NIAAA NIH HHS/United States
- R01 DA010072/DA/NIDA NIH HHS/United States
- DA023597/DA/NIDA NIH HHS/United States
- AA006420/AA/NIAAA NIH HHS/United States
- R01 AA008459/AA/NIAAA NIH HHS/United States
- DA004398/DA/NIDA NIH HHS/United States
- AA008459/AA/NIAAA NIH HHS/United States
- R01 DA004043/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

