Compartmentalization of immune responses during Staphylococcus aureus cranial bone flap infection
- PMID: 23747950
- PMCID: PMC3730773
- DOI: 10.1016/j.ajpath.2013.04.031
Compartmentalization of immune responses during Staphylococcus aureus cranial bone flap infection
Abstract
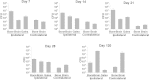
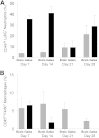
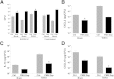
Decompressive craniectomy is often required after head trauma, stroke, or cranial bleeding to control subsequent brain swelling and prevent death. The infection rate after cranial bone flap replacement ranges from 0.8% to 15%, with an alarming frequency caused by methicillin-resistant Staphylococcus aureus, which is problematic because of recalcitrance to antibiotic therapy. Herein we report the establishment of a novel mouse model of S. aureus cranial bone flap infection that mimics several aspects of human disease. Bacteria colonized bone flaps for up to 4 months after infection, as revealed by scanning electron microscopy and quantitative culture, demonstrating the chronicity of the model. Analysis of a human cranial bone flap with confirmed S. aureus infection by scanning electron microscopy revealed similar structural attributes as the mouse model, demonstrating that it closely parallels structural facets of human disease. Inflammatory indices were most pronounced within the subcutaneous galeal compartment compared with the underlying brain parenchyma. Specifically, neutrophil influx and chemokine expression (CXCL2 and CCL5) were markedly elevated in the galea, which demonstrated substantial edema on magnetic resonance images, whereas the underlying brain parenchyma exhibited minimal involvement. Evaluation of immune mechanisms required for bacterial containment and inflammation revealed critical roles for MyD88-dependent signaling and neutrophils. This novel mouse model of cranial bone flap infection can be used to identify key immunologic and therapeutic mechanisms relevant to persistent bone flap infection in humans.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Bhaskar I.P., Zaw N.N., Zheng M., Lee G.Y. Bone flap storage following craniectomy: a survey of practices in major Australian neurosurgical centres. ANZ J Surg. 2011;81:137–141. - PubMed
-
- Dashti S.R., Baharvahdat H., Spetzler R.F., Sauvageau E., Chang S.W., Stiefel M.F., Park M.S., Bambakidis N.C. Operative intracranial infection following craniotomy. Neurosurg Focus. 2008;24:E10. - PubMed
-
- Baumeister S., Peek A., Friedman A., Levin L.S., Marcus J.R. Management of postneurosurgical bone flap loss caused by infection. Plast Reconstr Surg. 2008;122:195e–208e. - PubMed
-
- Hammon W.M., Kempe L.G. Methyl methacrylate cranioplasty: 13 years experience with 417 patients. Acta Neurochir (Wien) 1971;25:69–77. - PubMed
-
- Honeybul S., Ho K.M. Long-term complications of decompressive craniectomy for head injury. J Neurotrauma. 2011;28:929–935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

