Development and pilot testing of a video-assisted informed consent process
- PMID: 23747986
- PMCID: PMC3769445
- DOI: 10.1016/j.cct.2013.05.011
Development and pilot testing of a video-assisted informed consent process
Abstract
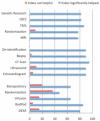
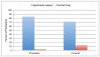
The informed consent process for research has come under scrutiny, as consent documents are increasingly long and difficult to understand. Innovations are needed to improve comprehension in order to make the consent process truly informed. We report on the development and pilot testing of video clips that could be used during the consent process to better explain research procedures to potential participants. Based on input from researchers and community partners, 15 videos of common research procedures/concepts were produced. The utility of the videos was then tested by embedding them in mock-informed consent documents that were presented via an online electronic consent system designed for delivery via iPad. Three mock consents were developed, each containing five videos. All participants (n = 61) read both a paper version and the video-assisted iPad version of the same mock consent and were randomized to which format they reviewed first. Participants were given a competency quiz that posed specific questions about the information in the consent after reviewing the first consent document to which they were exposed. Most participants (78.7%) preferred the video-assisted format compared to paper (12.9%). Nearly all (96.7%) reported that the videos improved their understanding of the procedures described in the consent document; however, the comprehension of material did not significantly differ by consent format. Results suggest videos may be helpful in providing participants with information about study procedures in a way that is easy to understand. Additional testing of video consents for complex protocols and with subjects of lower literacy is warranted.
Keywords: Electronic forms; Informed consent process; Tablet computing; Videos.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
References
-
- Fortun P, West J, Chalkley L, Shonde A, Hawkey C. Recall of informed consent information by healthy volunteers in clinical trials. QJM : monthly journal of the Association of Physicians. 2008;101:625–9. - PubMed
-
- Agoritsas T, Perneger TV. Patient-reported conformity of informed consent procedures and participation in clinical research. QJM : monthly journal of the Association of Physicians. 2011;104:151–9. - PubMed
-
- Dunn LB, Lindamer LA, Palmer BW, Schneiderman LJ, Jeste DV. Enhancing comprehension of consent for research in older patients with psychosis: a randomized study of a novel consent procedure. The American journal of psychiatry. 2001;158:1911–3. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources