Effects of non-linearity on cell-ECM interactions
- PMID: 23748051
- PMCID: PMC3930572
- DOI: 10.1016/j.yexcr.2013.05.017
Effects of non-linearity on cell-ECM interactions
Abstract
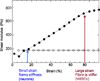
Filamentous biopolymers such as F-actin, vimentin, fibrin and collagen that form networks within the cytoskeleton or the extracellular matrix have unusual rheological properties not present in most synthetic soft materials that are used as cell substrates or scaffolds for tissue engineering. Gels formed by purified filamentous biopolymers are often strain stiffening, with an elastic modulus that can increase an order of magnitude at moderate strains that are relevant to cell and tissue deformation in vivo. This review summarizes some experimental studies of non-linear rheology in biopolymer gels, discusses possible molecular mechanisms that account for strain stiffening, and explores the possible relevance of non-linear rheology to the interactions between cell and extracellular matrices.
Keywords: cell; cell-ECM interaction; extracellular matrix; non-linear elasticity; strain-stiffening.
© 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells.Nature. 2019 Sep;573(7772):96-101. doi: 10.1038/s41586-019-1516-5. Epub 2019 Aug 28. Nature. 2019. PMID: 31462779
-
Uncoupling shear and uniaxial elastic moduli of semiflexible biopolymer networks: compression-softening and stretch-stiffening.Sci Rep. 2016 Jan 13;6:19270. doi: 10.1038/srep19270. Sci Rep. 2016. PMID: 26758452 Free PMC article.
-
Functional mechanical attributes of natural and synthetic gel-based scaffolds in tissue engineering: strain-stiffening effects on apparent elastic modulus and compressive toughness.J Mech Behav Biomed Mater. 2022 Feb;126:105066. doi: 10.1016/j.jmbbm.2021.105066. Epub 2022 Jan 5. J Mech Behav Biomed Mater. 2022. PMID: 35008012
-
Hybrid and Composite Scaffolds Based on Extracellular Matrices for Cartilage Tissue Engineering.Tissue Eng Part B Rev. 2019 Jun;25(3):202-224. doi: 10.1089/ten.TEB.2018.0245. Tissue Eng Part B Rev. 2019. PMID: 30648478 Review.
-
Biochemical and biophysical aspects of collagen nanostructure in the extracellular matrix.Physiol Res. 2007;56 Suppl 1:S51-S60. doi: 10.33549/physiolres.931302. Epub 2007 May 31. Physiol Res. 2007. PMID: 17552894 Review.
Cited by
-
Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components.Genes (Basel). 2023 Jan 20;14(2):272. doi: 10.3390/genes14020272. Genes (Basel). 2023. PMID: 36833199 Free PMC article. Review.
-
Physics of collective cell migration.Eur Biophys J. 2023 Nov;52(8):625-640. doi: 10.1007/s00249-023-01681-w. Epub 2023 Sep 14. Eur Biophys J. 2023. PMID: 37707627 Review.
-
Collagen microarchitecture mechanically controls myofibroblast differentiation.Proc Natl Acad Sci U S A. 2020 May 26;117(21):11387-11398. doi: 10.1073/pnas.1919394117. Epub 2020 May 8. Proc Natl Acad Sci U S A. 2020. PMID: 32385149 Free PMC article.
-
Fibrin mechanical properties and their structural origins.Matrix Biol. 2017 Jul;60-61:110-123. doi: 10.1016/j.matbio.2016.08.003. Epub 2016 Aug 20. Matrix Biol. 2017. PMID: 27553509 Free PMC article. Review.
-
Influence of Temperature and Polymer Concentration on the Nonlinear Response of Highly Acetylated Chitosan-Genipin Hydrogels.Gels. 2022 Mar 21;8(3):194. doi: 10.3390/gels8030194. Gels. 2022. PMID: 35323307 Free PMC article.
References
-
- Fung YC. Elasticity of soft tissues in simple elongation. The American journal of physiology. 1967;213:1532–1544. - PubMed
-
- Shadwick RE. Mechanical design in arteries. The Journal of experimental biology. 1999;202:3305–3313. - PubMed
-
- Janmey PA, Amis EJ, Ferry JD. Viscoelastic properties of fibrin clots in large shearing deformations. Journal of Rheology. 1982;26:599–600.
-
- Motte S, Kaufman LJ. Strain stiffening in collagen I networks. Biopolymers. 2013;99:35–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

