Sulfenic acid chemistry, detection and cellular lifetime
- PMID: 23748139
- PMCID: PMC4184475
- DOI: 10.1016/j.bbagen.2013.05.040
Sulfenic acid chemistry, detection and cellular lifetime
Abstract
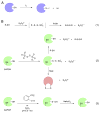
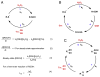
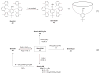
Background: Reactive oxygen species-mediated cysteine sulfenic acid modification has emerged as an important regulatory mechanism in cell signaling. The stability of sulfenic acid in proteins is dictated by the local microenvironment and ability of antioxidants to reduce this modification. Several techniques for detecting this cysteine modification have been developed, including direct and in situ methods.
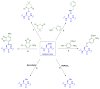
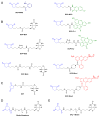
Scope of review: This review presents a historical discussion of sulfenic acid chemistry and highlights key examples of this modification in proteins. A comprehensive survey of available detection techniques with advantages and limitations is discussed. Finally, issues pertaining to rates of sulfenic acid formation, reduction, and chemical trapping methods are also covered.
Major conclusions: Early chemical models of sulfenic acid yielded important insights into the unique reactivity of this species. Subsequent pioneering studies led to the characterization of sulfenic acid formation in proteins. In parallel, the discovery of oxidant-mediated cell signaling pathways and pathological oxidative stress has led to significant interest in methods to detect these modifications. Advanced methods allow for direct chemical trapping of protein sulfenic acids directly in cells and tissues. At the same time, many sulfenic acids are short-lived and the reactivity of current probes must be improved to sample these species, while at the same time, preserving their chemical selectivity. Inhibitors with binding scaffolds can be rationally designed to target sulfenic acid modifications in specific proteins.
General significance: Ever increasing roles for protein sulfenic acids have been uncovered in physiology and pathology. A more complete understanding of sulfenic acid-mediated regulatory mechanisms will continue to require rigorous and new chemical insights. This article is part of a Special Issue entitled Current methods to study reactive oxygen species - pros and cons and biophysics of membrane proteins. Guest Editor: Christine Winterbourn.
Keywords: Cellular lifetimes of sulfenic acid; Sulfenic acid; Sulfenic acid chemistry; Sulfenic acid detection method.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
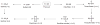










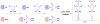
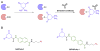
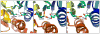


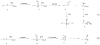



References
-
- Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol. 2000;17:1232–1239. - PubMed
-
- Pe’er I, Felder CE, Man O, Silman I, Sussman JL, Beckmann JS. Proteomic signatures: amino acid and oligopeptide compositions differentiate among phyla. Proteins Struct Funct Bioinform. 2004;54:20–40. - PubMed
-
- Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

