Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21
- PMID: 23748970
- PMCID: PMC3709480
- DOI: 10.1038/ncomms2978
Haemodynamically dependent valvulogenesis of zebrafish heart is mediated by flow-dependent expression of miR-21
Abstract
Heartbeat is required for normal development of the heart, and perturbation of intracardiac flow leads to morphological defects resembling congenital heart diseases. These observations implicate intracardiac haemodynamics in cardiogenesis, but the signalling cascades connecting physical forces, gene expression and morphogenesis are largely unknown. Here we use a zebrafish model to show that the microRNA, miR-21, is crucial for regulation of heart valve formation. Expression of miR-21 is rapidly switched on and off by blood flow. Vasoconstriction and increasing shear stress induce ectopic expression of miR-21 in the head vasculature and heart. Flow-dependent expression of mir-21 governs valvulogenesis by regulating the expression of the same targets as mouse/human miR-21 (sprouty, pdcd4, ptenb) and induces cell proliferation in the valve-forming endocardium at constrictions in the heart tube where shear stress is highest. We conclude that miR-21 is a central component of a flow-controlled mechanotransduction system in a physicogenetic regulatory loop.
Figures

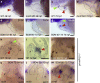
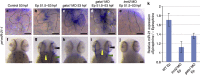

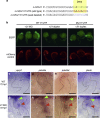

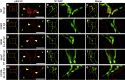
References
-
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 126, 1037–1048 (2006). - PubMed
-
- Stainier D. Y. Zebrafish genetics and vertebrate heart formation. Nat. Rev. Genet. 2, 39–48 (2001). - PubMed
-
- Forouhar A. S. et al. The embryonic vertebrate heart tube is a dynamic suction pump. Science 312, 751–753 (2006). - PubMed
-
- Hove J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421, 172–177 (2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

