Wnt and lithium: a common destiny in the therapy of nervous system pathologies?
- PMID: 23749084
- PMCID: PMC11113114
- DOI: 10.1007/s00018-013-1378-1
Wnt and lithium: a common destiny in the therapy of nervous system pathologies?
Abstract
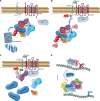
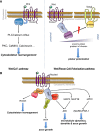
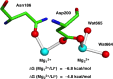
Wnt signaling is required for neurogenesis, the fate of neural progenitors, the formation of neuronal circuits during development, neuron positioning and polarization, axon and dendrite development and finally for synaptogenesis. This signaling pathway is also implicated in the generation and differentiation of glial cells. In this review, we describe the mechanisms of action of Wnt signaling pathways and their implication in the development and correct functioning of the nervous system. We also illustrate how a dysregulated Wnt pathway could lead to psychiatric, neurodegenerative and demyelinating pathologies. Lithium, used for the treatment of bipolar disease, inhibits GSK3β, a central enzyme of the Wnt/β-catenin pathway. Thus, lithium could, to some extent, mimic Wnt pathway. We highlight the possible dialogue between lithium therapy and modulation of Wnt pathway in the treatment of the diseases of the nervous system.
Figures
References
-
- Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6(5):351–362. - PubMed
-
- Carter M, Chen X, Slowinska B, Minnerath S, Glickstein S, Shi L, Campagne F, Weinstein H, Ross ME. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc Natl Acad Sci USA. 2005;102(36):12843–12848. - PMC - PubMed
-
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in xenopus. Development. 2001;128(21):4189–4201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

