XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling
- PMID: 23749209
- PMCID: PMC3746193
- DOI: 10.1038/emboj.2013.133
XIAP inhibits autophagy via XIAP-Mdm2-p53 signalling
Abstract
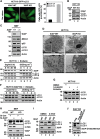
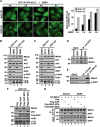
The primary role of autophagy is adaption to starvation. However, increasing evidence suggests that autophagy inhibition also plays an important role in tumorigenesis. Upregulation of X-linked inhibitor of apoptosis (XIAP) has been associated to a variety of human cancers, yet the underlying mechanisms remain obscure. Here, we report that XIAP suppresses autophagy by exerting a previously unidentified ubiquitin E3 ligase activity towards Mdm2, which is a negative regulator of p53. XIAP controls serum starvation-induced autophagy downstream of the PI3K/Akt pathway. In mouse models, inhibition of autophagy by XIAP promotes tumorigenecity of HCT116 cells. XIAP-mediated autophagy inhibition is also largely validated in clinical tumour samples. These findings reveal a novel XIAP-Mdm2-p53 pathway that mediates the inhibition of autophagy, by which XIAP may contribute to tumorigenesis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Comment in
-
XIAP: inhibitor of two worlds.EMBO J. 2013 Aug 14;32(16):2187-8. doi: 10.1038/emboj.2013.152. Epub 2013 Jun 21. EMBO J. 2013. PMID: 23792426 Free PMC article.
References
-
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B (1999) Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics 59: 59–65 - PubMed
-
- Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, Ryan KM (2006) DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126: 121–134 - PubMed
-
- Damgaard RB, Nachbur U, Yabal M, Wong WW, Fiil BK, Kastirr M, Rieser E, Rickard JA, Bankovacki A, Peschel C, Ruland J, Bekker-Jensen S, Mailand N, Kaufmann T, Strasser A, Walczak H, Silke J, Jost PJ, Gyrd-Hansen M (2012) The ubiquitin ligase XIAP recruits LUBAC for NOD2 signaling in inflammation and innate immunity. Mol Cell 46: 746–758 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

