Probing the boundaries of orthology: the unanticipated rapid evolution of Drosophila centrosomin
- PMID: 23749319
- PMCID: PMC3730919
- DOI: 10.1534/genetics.113.152546
Probing the boundaries of orthology: the unanticipated rapid evolution of Drosophila centrosomin
Abstract
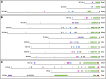
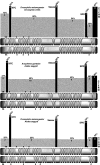
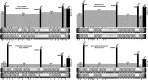
The rapid evolution of essential developmental genes and their protein products is both intriguing and problematic. The rapid evolution of gene products with simple protein folds and a lack of well-characterized functional domains typically result in a low discovery rate of orthologous genes. Additionally, in the absence of orthologs it is difficult to study the processes and mechanisms underlying rapid evolution. In this study, we have investigated the rapid evolution of centrosomin (cnn), an essential gene encoding centrosomal protein isoforms required during syncytial development in Drosophila melanogaster. Until recently the rapid divergence of cnn made identification of orthologs difficult and questionable because Cnn violates many of the assumptions underlying models for protein evolution. To overcome these limitations, we have identified a group of insect orthologs and present conserved features likely to be required for the functions attributed to cnn in D. melanogaster. We also show that the rapid divergence of Cnn isoforms is apparently due to frequent coding sequence indels and an accelerated rate of intronic additions and eliminations. These changes appear to be buffered by multi-exon and multi-reading frame maximum potential ORFs, simple protein folds, and the splicing machinery. These buffering features also occur in other genes in Drosophila and may help prevent potentially deleterious mutations due to indels in genes with large coding exons and exon-dense regions separated by small introns. This work promises to be useful for future investigations of cnn and potentially other rapidly evolving genes and proteins.
Keywords: Cnn; Drosophila; centrosome; indels; rapid evolution.
Figures







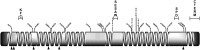


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

