Enhanced radiation sensitivity in HPV-positive head and neck cancer
- PMID: 23749640
- PMCID: PMC3732540
- DOI: 10.1158/0008-5472.CAN-13-0587
Enhanced radiation sensitivity in HPV-positive head and neck cancer
Abstract
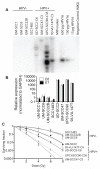
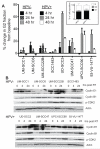
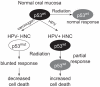
Patients with human papillomavirus (HPV+)-associated head and neck cancer (HNC) show significantly improved survival outcome compared with those with HPV-negative (HPV-) tumors. Published data examining this difference offers conflicting results to date. We systematically investigated the radiation sensitivity of all available validated HPV+ HNC cell lines and a series of HPV- HNC cell lines using in vitro and in vivo techniques. HPV+ HNCs exhibited greater intrinsic radiation sensitivity (average SF2 HPV-: 0.59 vs. HPV+: 0.22; P < 0.0001), corresponding with a prolonged G2-M cell-cycle arrest and increased apoptosis following radiation exposure (percent change 0% vs. 85%; P = 0.002). A genome-wide microarray was used to compare gene expression 24 hours following radiation between HPV+ and HPV- cell lines. Multiple genes in TP53 pathway were upregulated in HPV+ cells (Z score 4.90), including a 4.6-fold increase in TP53 (P < 0.0001). Using immortalized human tonsillar epithelial (HTE) cells, increased radiation sensitivity was seen in cell expressing HPV-16 E6 despite the effect of E6 to degrade p53. This suggested that low levels of normally functioning p53 in HPV+ HNC cells could be activated by radiation, leading to cell death. Consistent with this, more complete knockdown of TP53 by siRNA resulted in radiation resistance. These results provide clear evidence, and a supporting mechanism, for increased radiation sensitivity in HPV+ HNC relative to HPV- HNC. This issue is under active investigation in a series of clinical trials attempting to de-escalate radiation (and chemotherapy) in selected patients with HPV+ HNC in light of their favorable overall survival outcome.
©2013 AACR.
Figures
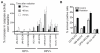

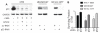

References
-
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. - PubMed
-
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence Trends for Human Papillomavirus-Related and -Unrelated Oral Squamous Cell Carcinomas in the United States. J Clin Oncol. 2008;26:612–9. - PubMed
-
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved Survival of Patients With Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma in a Prospective Clinical Trial. J Natl Cancer Inst. 2008;100:261–9. - PubMed
-
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

