Gefitinib selectively inhibits tumor cell migration in EGFR-amplified human glioblastoma
- PMID: 23749785
- PMCID: PMC3714155
- DOI: 10.1093/neuonc/not053
Gefitinib selectively inhibits tumor cell migration in EGFR-amplified human glioblastoma
Abstract
Background: Tissue invasion is a hallmark of most human cancers and remains a major source of treatment failure in patients with glioblastoma (GBM). Although EGFR amplification has been previously associated with more invasive tumor behavior, existing experimental models have not supported quantitative evaluation of interpatient differences in tumor cell migration or testing of patient-specific responses to therapies targeting invasion. To explore these questions, we optimized an ex vivo organotypic slice culture system allowing for labeling and tracking of tumor cells in human GBM slice cultures.
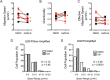
Methods: With use of time-lapse confocal microscopy of retrovirally labeled tumor cells in slices, baseline differences in migration speed and efficiency were determined and correlated with EGFR amplification in a cohort of patients with GBM. Slices were treated with gefitinib to evaluate anti-invasive effects associated with targeting EGFR.
Results: Migration analysis identified significant patient-to-patient variation at baseline. EGFR amplification was correlated with increased migration speed and efficiency compared with nonamplified tumors. Critically, gefitinib resulted in a selective and significant reduction of tumor cell migration in EGFR-amplified tumors.
Conclusions: These data provide the first identification of patient-to-patient variation in tumor cell migration in living human tumor tissue. We found that EGFR-amplified GBM are inherently more efficient in their migration and can be effectively targeted by gefitinib treatment. These data suggest that stratified clinical trails are needed to evaluate gefitinib as an anti-invasive adjuvant for patients with EGFR-amplified GBM. In addition, these results provide proof of principle that primary slice cultures may be useful for patient-specific screening of agents designed to inhibit tumor invasion.
Keywords: EGFR; gefitinib; glioblastoma; invasion; migration; personalized therapy; slice culture.
Figures



References
-
- Gaspar LE, Fisher BJ, Macdonald DR, et al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24(1):55–57. - PubMed
-
- Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. - PubMed
-
- Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

