Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors
- PMID: 23749810
- PMCID: PMC3730084
- DOI: 10.1093/pcp/pct086
Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors
Abstract
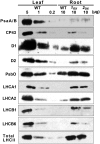
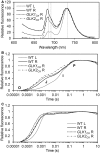
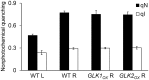
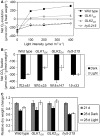
In plants, genes involved in photosynthesis are encoded separately in nuclei and plastids, and tight cooperation between these two genomes is therefore required for the development of functional chloroplasts. Golden2-like (GLK) transcription factors are involved in chloroplast development, directly targeting photosynthesis-associated nuclear genes for up-regulation. Although overexpression of GLKs leads to chloroplast development in non-photosynthetic organs, the mechanisms of coordination between the nuclear gene expression influenced by GLKs and the photosynthetic processes inside chloroplasts are largely unknown. To elucidate the impact of GLK-induced expression of photosynthesis-associated nuclear genes on the construction of photosynthetic systems, chloroplast morphology and photosynthetic characteristics in greenish roots of Arabidopsis thaliana lines overexpressing GLKs were compared with those in wild-type roots and leaves. Overexpression of GLKs caused up-regulation of not only their direct targets but also non-target nuclear and plastid genes, leading to global induction of chloroplast biogenesis in the root. Large antennae relative to reaction centers were observed in wild-type roots and were further enhanced by GLK overexpression due to the increased expression of target genes associated with peripheral light-harvesting antennae. Photochemical efficiency was lower in the root chloroplasts than in leaf chloroplasts, suggesting that the imbalance in the photosynthetic machinery decreases the efficiency of light utilization in root chloroplasts. Despite the low photochemical efficiency, root photosynthesis contributed to carbon assimilation in Arabidopsis. Moreover, GLK overexpression increased CO₂ fixation and promoted phototrophic performance of the root, showing the potential of root photosynthesis to improve effective carbon utilization in plants.
Keywords: Arabidopsis root; Chloroplast development; Construction of photosynthetic systems; GLK; Photosynthesis.
Figures






Similar articles
-
GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis.Plant Cell. 2009 Apr;21(4):1109-28. doi: 10.1105/tpc.108.065250. Epub 2009 Apr 17. Plant Cell. 2009. PMID: 19376934 Free PMC article.
-
Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis.Plant Cell. 2012 Mar;24(3):1081-95. doi: 10.1105/tpc.111.092254. Epub 2012 Mar 13. Plant Cell. 2012. PMID: 22415275 Free PMC article.
-
Application of HB17, an Arabidopsis class II homeodomain-leucine zipper transcription factor, to regulate chloroplast number and photosynthetic capacity.J Exp Bot. 2013 Nov;64(14):4479-90. doi: 10.1093/jxb/ert261. Epub 2013 Sep 4. J Exp Bot. 2013. PMID: 24006420 Free PMC article.
-
[Biological function and molecular mechanism of the transcription factor GLKs in plants: a review].Sheng Wu Gong Cheng Xue Bao. 2022 Aug 25;38(8):2700-2712. doi: 10.13345/j.cjb.220099. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 36002404 Review. Chinese.
-
Chloroplast development in green plant tissues: the interplay between light, hormone, and transcriptional regulation.New Phytol. 2022 Mar;233(5):2000-2016. doi: 10.1111/nph.17839. Epub 2021 Nov 24. New Phytol. 2022. PMID: 34729790 Review.
Cited by
-
Identification of GOLDEN2-like transcription factor genes in soybeans and their role in regulating plant development and metal ion stresses.Front Plant Sci. 2022 Nov 11;13:1052659. doi: 10.3389/fpls.2022.1052659. eCollection 2022. Front Plant Sci. 2022. PMID: 36438095 Free PMC article.
-
Integrative analysis of hexaploid wheat roots identifies signature components during iron starvation.J Exp Bot. 2019 Nov 18;70(21):6141-6161. doi: 10.1093/jxb/erz358. J Exp Bot. 2019. PMID: 31738431 Free PMC article.
-
Carotenoid Biosynthesis and Plastid Development in Plants: The Role of Light.Int J Mol Sci. 2021 Jan 26;22(3):1184. doi: 10.3390/ijms22031184. Int J Mol Sci. 2021. PMID: 33530294 Free PMC article. Review.
-
ZmDof08, a zinc finger transcription factor, plays critical roles in photosynthesis in maize.Plant Cell Rep. 2025 Sep 3;44(9):207. doi: 10.1007/s00299-025-03591-x. Plant Cell Rep. 2025. PMID: 40903564
-
Rice straw-derived smoke water promotes rice root growth under phosphorus deficiency by modulating oxidative stress and photosynthetic gene expression.Sci Rep. 2023 Sep 8;13(1):14802. doi: 10.1038/s41598-023-41987-5. Sci Rep. 2023. PMID: 37684292 Free PMC article.
References
-
- Aschan G, Pfanz H. Non-foliar photosynthesis—a strategy of additional carbon acquisition. Flora. 2003;198:81–97.
-
- De Santis-MacIossek G, Kofer W, Bock A, Schoch S, Maier RM, Wanner G, et al. Targeted disruption of the plastid RNA polymerase genes rpoA, B and C1: molecular biology, biochemistry and ultrastructure. Plant J. 1999;18:477–489. - PubMed
-
- Fitter DW, Martin DJ, Copley MJ, Scotland RW, Langdale JA. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002;31:713–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

