Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection
- PMID: 23749840
- PMCID: PMC3756431
- DOI: 10.1136/thoraxjnl-2012-202230
Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection
Abstract
Rationale: Arikace is a liposomal amikacin preparation for aerosol delivery with potent Pseudomonas aeruginosa killing and prolonged lung deposition.
Objectives: To examine the safety and efficacy of 28 days of once-daily Arikace in cystic fibrosis (CF) patients chronically infected with P aeruginosa.
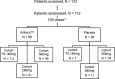
Methods: 105 subjects were evaluated in double-blind, placebo-controlled studies. Subjects were randomised to once-daily Arikace (70, 140, 280 and 560 mg; n=7, 5, 21 and 36 subjects) or placebo (n=36) for 28 days. Primary outcomes included safety and tolerability. Secondary outcomes included lung function (forced expiratory volume at one second (FEV1)), P aeruginosa density in sputum, and the Cystic Fibrosis Quality of Life Questionnaire-Revised (CFQ-R).
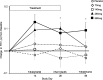
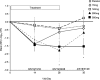
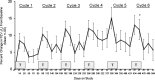
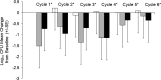
Results: The adverse event profile was similar among Arikace and placebo subjects. The relative change in FEV1 was higher in the 560 mg dose group at day 28 (p=0.033) and at day 56 (28 days post-treatment, 0.093L±0.203 vs -0.032L±0.119; p=0.003) versus placebo. Sputum P aeruginosa density decreased >1 log in the 560 mg group versus placebo (days 14, 28 and 35; p=0.021). The Respiratory Domain of the CFQ-R increased by the Minimal Clinically Important Difference (MCID) in 67% of Arikace subjects (560 mg) versus 36% of placebo (p=0.006), and correlated with FEV1 improvements at days 14, 28 and 42 (p<0.05). An open-label extension (560 mg Arikace) for 28 days followed by 56 days off over six cycles confirmed durable improvements in lung function and sputum P aeruginosa density (n=49).
Conclusions: Once-daily Arikace demonstrated acute tolerability, safety, biologic activity and efficacy in patients with CF with P aeruginosa infection.
Trial registration: ClinicalTrials.gov NCT00558844 NCT00777296.
Keywords: Bacterial Infection; Cystic Fibrosis; Respiratory Infection.
Figures
References
-
- Rommens JM, Iannuzzi MC, Kerem B, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science (New York, NY) 1989;245:1059–65 - PubMed
-
- Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science (New York, NY) 1989;245:1073–80 - PubMed
-
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001 - PubMed
-
- Strausbaugh SD, Davis PB. Cystic fibrosis: a review of epidemiology and pathobiology. Clin Chest Med 2007;28:279–88 - PubMed
-
- Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005;293:581–8 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
