Ion selectivity from local configurations of ligands in solutions and ion channels
- PMID: 23750043
- PMCID: PMC3674792
- DOI: 10.1016/j.cplett.2009.12.013
Ion selectivity from local configurations of ligands in solutions and ion channels
Abstract
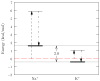
Probabilities of numbers of ligands proximal to an ion lead to simple, general formulae for the free energy of ion selectivity between different media. That free energy does not depend on the definition of an inner shell for ligand-counting, but other quantities of mechanistic interest do. If analysis is restricted to a specific coordination number, then two distinct probabilities are required to obtain the free energy in addition. The normalizations of those distributions produce partition function formulae for the free energy. Quasi-chemical theory introduces concepts of chemical equilibrium, then seeks the probability that is simplest to estimate, that of the most probable coordination number. Quasi-chemical theory establishes the utility of distributions of ligand-number, and sharpens our understanding of quasi-chemical calculations based on electronic structure methods. This development identifies contributions with clear physical interpretations, and shows that evaluation of those contributions can establish a mechanistic understanding of the selectivity in ion channels.
Figures




References
-
- Rempe SB, Pratt LR, Hummer G, Kress JD, Martin RL, Redondo A. J Am Chem Soc. 2000;122:966.
-
- Mason P, Ansell S, Neilson G. J Phys: Condens Matter. 2006;18:8437. - PubMed
-
- Noskov SY, Bernèche S, Roux B. Nature. 2004;431:830. - PubMed
-
- Asthagiri D, Pratt LR, Paulaitis ME. J Chem Phys. 2006;125:024701. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
