Altered functionality of anti-bacterial antibodies in patients with chronic hepatitis C virus infection
- PMID: 23750224
- PMCID: PMC3672197
- DOI: 10.1371/journal.pone.0064992
Altered functionality of anti-bacterial antibodies in patients with chronic hepatitis C virus infection
Abstract
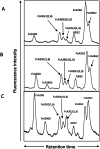
Background: Using comparative glycoproteomics, we have previously identified a glycoprotein that is altered in both amount and glycosylation as a function of liver cirrhosis. The altered glycoprotein is an agalactosylated (G0) immunoglobulin G molecule (IgG) that recognizes the heterophilic alpha-gal epitope. Since the alpha gal epitope is found on gut enterobacteria, it has been hypothesized that anti-gal antibodies are generated as a result of increased bacterial exposure in patients with liver disease.
Methods: The N-linked glycosylation of anti-gal IgG molecules from patients with fibrosis and cirrhosis was determined and the effector function of anti-bacterial antibodies from over 100 patients examined. In addition, markers of microbial exposure were determined.
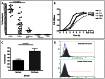
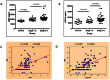
Results: Surprisingly, the subset of agalactosylated anti-gal antibodies described here, was impaired in their ability to mediate complement mediated lysis and inhibited the complement-mediated destruction of common gut bacteria. In an analysis of serum from more than 100 patients with liver disease, we have shown that those with increased levels of this modified anti-gal antibody had increased levels of markers of bacterial exposure.
Conclusions: Anti-gal antibodies in patients with liver cirrhosis were reduced in their ability to mediate complement mediated lysis of target cells. As bacterial infection is a major complication in patients with cirrhosis and bacterial products such as LPS are thought to play a major role in the development and progression of liver fibrosis, this finding has many clinical implications in the etiology, prognosis and treatment of liver disease.
Conflict of interest statement
Figures



References
-
- Alter MJ (1997) Epidemiology of hepatitis C. Hepatology. 26: 62S–65S. - PubMed
-
- Hoofnagle JH (1997) Hepatitis C: the clinical spectrum of disease. Hepatology 26: 15S–20S. - PubMed
-
- Di Bisceglie AM (1997) Hepatitis C and hepatocellular carcinoma. Hepatology 26: 34S–38S. - PubMed
-
- Friedman SL (2007) A deer in the headlights: BAMBI meets liver fibrosis. Nat Med 13: 1281–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

