TWEAK-independent Fn14 self-association and NF-κB activation is mediated by the C-terminal region of the Fn14 cytoplasmic domain
- PMID: 23750247
- PMCID: PMC3672086
- DOI: 10.1371/journal.pone.0065248
TWEAK-independent Fn14 self-association and NF-κB activation is mediated by the C-terminal region of the Fn14 cytoplasmic domain
Abstract
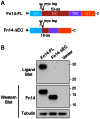
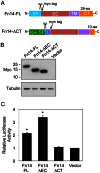
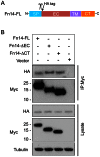
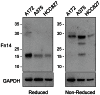
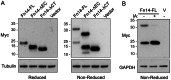
The tumor necrosis factor (TNF) superfamily member TNF-like weak inducer of apoptosis (TWEAK) is a pro-inflammatory and pro-angiogenic cytokine implicated in physiological tissue regeneration and wound repair. TWEAK binds to a 102-amino acid type I transmembrane cell surface receptor named fibroblast growth factor-inducible 14 (Fn14). TWEAK:Fn14 engagement activates several intracellular signaling cascades, including the NF-κB pathway, and sustained Fn14 signaling has been implicated in the pathogenesis of chronic inflammatory diseases and cancer. Although several groups are developing TWEAK- or Fn14-targeted agents for therapeutic use, much more basic science research is required before we fully understand the TWEAK/Fn14 signaling axis. For example, we and others have proposed that TWEAK-independent Fn14 signaling may occur in cells when Fn14 levels are highly elevated, but this idea has never been tested directly. In this report, we first demonstrate TWEAK-independent Fn14 signaling by showing that an Fn14 deletion mutant that is unable to bind TWEAK can activate the NF-κB pathway in transfected cells. We then show that ectopically-expressed, cell surface-localized Fn14 can self-associate into Fn14 dimers, and we show that Fn14 self-association is mediated by an 18-aa region within the Fn14 cytoplasmic domain. Endogenously-expressed Fn14 as well as ectopically-overexpressed Fn14 could also be detected in dimeric form when cell lysates were subjected to SDS-PAGE under non-reducing conditions. Additional experiments revealed that Fn14 dimerization occurs during cell lysis via formation of an intermolecular disulfide bond at cysteine residue 122. These findings provide insight into the Fn14 signaling mechanism and may aid current studies to develop therapeutic agents targeting this small cell surface receptor.
Conflict of interest statement
Figures


References
-
- Meighan-Mantha RL, Hsu DKW, Guo Y, Brown SAN, Feng SY, et al. (1999) The mitogen-inducible Fn14 gene encodes a type I transmembrane protein that modulates fibroblast adhesion and migration. J Biol Chem 274: 33166–33176. - PubMed
-
- Wiley SR, Cassiano L, Lofton T, Davis-Smith T, Winkles JA, et al. (2001) A novel TNF receptor family member binds TWEAK and is implicated in angiogenesis. Immunity 15: 837–846. - PubMed
-
- Burkly LC, Michaelson JS, Zheng TS (2011) TWEAK/Fn14 pathway: An immunological switch for shaping tissue responses. Immunol Rev 244: 99–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

