Whole blood stimulation with Toll-like receptor (TLR)-7/8 and TLR-9 agonists induces interleukin-12p40 expression in plasmacytoid dendritic cells in rhesus macaques but not in humans
- PMID: 23750720
- PMCID: PMC3784223
- DOI: 10.1111/cei.12155
Whole blood stimulation with Toll-like receptor (TLR)-7/8 and TLR-9 agonists induces interleukin-12p40 expression in plasmacytoid dendritic cells in rhesus macaques but not in humans
Abstract
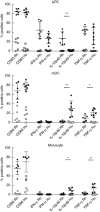
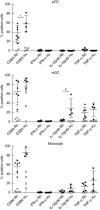
Macaques provide important animal models in biomedical research into infectious and chronic inflammatory disease. Therefore, a proper understanding of the similarities and differences in immune function between macaques and humans is needed for adequate interpretation of the data and translation to the human situation. Dendritic cells are important as key regulators of innate and adaptive immune responses. Using a new whole blood assay we investigated functional characteristics of blood plasmacytoid dendritic cells (pDC), myeloid dendritic cells (mDC) and monocytes in rhesus macaques by studying induction of activation markers and cytokine expression upon Toll-like receptor (TLR) stimulation. In a head-to-head comparison we observed that rhesus macaque venous blood contained relatively lower numbers of pDC than human venous blood, while mDC and monocytes were present at similar percentages. In contrast to humans, pDC in rhesus macaques expressed the interleukin (IL)-12p40 subunit in response to TLR-7/8 as well as TLR-9 stimulation. Expression of IL-12p40 was confirmed by using different monoclonal antibodies and by reverse transcription-polymerase chain reaction (RT-PCR). Both in humans and rhesus macaques, TLR-4 stimulation induced IL-12p40 expression in mDC and monocytes, but not in pDC. The data show that, in contrast to humans, pDC in macaques are able to express IL-12p40, which could have consequences for evaluation of human vaccine candidates and viral infection.
Keywords: Toll-like receptors (TLRs); animal models - non-human primates; cytokine/interleukin/chemokine receptors; dendritic cells (myeloid; monocyte-derived); plasmacytoid.
© 2013 British Society for Immunology.
Figures






References
-
- Bontrop RE. Non-human primates: essential partners in biomedical research. Immunol Rev. 2001;183:5–9. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Lozach PY, Lortat-Jacob H, de Lacroix de Lavalette A, et al. DC-SIGN and L-SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem. 2003;278:20358–20366. - PubMed
-
- Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells [see comments] Cell. 2000;100:587–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

