Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide
- PMID: 23751126
- PMCID: PMC4089357
- DOI: 10.1517/17425247.2013.808184
Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: crossing the blood-brain barrier divide
Erratum in
- Expert Opin Drug Deliv. 2013 Oct;10(10):1463
Abstract
Introduction: Brain tumors are inherently difficult to treat in large part due to the cellular blood-brain barriers (BBBs) that limit the delivery of therapeutics to the tumor tissue from the systemic circulation. Virtually no large molecules, including antibody-based proteins, can penetrate the BBB. With antibodies fast becoming attractive ligands for highly specific molecular targeting to tumor antigens, a variety of methods are being investigated to enhance the access of these agents to intracranial tumors for imaging or therapeutic applications.
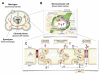
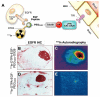
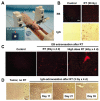
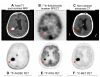
Areas covered: This review describes the characteristics of the BBB and the vasculature in brain tumors, described as the blood-brain tumor barrier (BBTB). Antibodies targeted to molecular markers of central nervous system (CNS) tumors will be highlighted, and current strategies for enhancing the delivery of antibodies across these cellular barriers into the brain parenchyma to the tumor will be discussed. Noninvasive imaging approaches to assess BBB/BBTB permeability and/or antibody targeting will be presented as a means of guiding the optimal delivery of targeted agents to brain tumors.
Expert opinion: Preclinical and clinical studies highlight the potential of several approaches in increasing brain tumor delivery across the BBB divide. However, each carries its own risks and challenges. There is tremendous potential in using neuroimaging strategies to assist in understanding and defining the challenges to translating and optimizing molecularly targeted antibody delivery to CNS tumors to improve clinical outcomes.
Figures

References
-
- CBTRUS . CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2008. Central Brain Tumor Registry of the United States; Hinsdale, IL: 2012.
-
- Gurney JG, Smith MA, Bunin GR. Chapter III: CNS and miscellaneous intracranial and intraspinal neoplasms. In: Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program; Bethesda, MD: 1999.
-
- Bernstein M, Berger MS. Neuro-oncology: the essentials. 2nd ed. Thieme Medical Publishers, Inc.; New York: 2008.
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
