N-linked protein glycosylation in the endoplasmic reticulum
- PMID: 23751184
- PMCID: PMC3721281
- DOI: 10.1101/cshperspect.a013359
N-linked protein glycosylation in the endoplasmic reticulum
Abstract
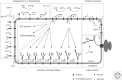
The attachment of glycans to asparagine residues of proteins is an abundant and highly conserved essential modification in eukaryotes. The N-glycosylation process includes two principal phases: the assembly of a lipid-linked oligosaccharide (LLO) and the transfer of the oligosaccharide to selected asparagine residues of polypeptide chains. Biosynthesis of the LLO takes place at both sides of the endoplasmic reticulum (ER) membrane and it involves a series of specific glycosyltransferases that catalyze the assembly of the branched oligosaccharide in a highly defined way. Oligosaccharyltransferase (OST) selects the Asn-X-Ser/Thr consensus sequence on polypeptide chains and generates the N-glycosidic linkage between the side-chain amide of asparagine and the oligosaccharide. This ER-localized pathway results in a systemic modification of the proteome, the basis for the Golgi-catalyzed modification of the N-linked glycans, generating the large diversity of N-glycoproteome in eukaryotic cells. This article focuses on the processes in the ER. Based on the highly conserved nature of this pathway we concentrate on the mechanisms in the eukaryotic model organism Saccharomyces cerevisiae.
Figures

References
-
- Adair WL Jr, Cafmeyer N 1987. Characterization of the Saccharomyces cerevisiae cis-prenyltransferase required for dolichyl phosphate biosynthesis. Arch Biochem Biophys 259: 589–596 - PubMed
-
- Aebi M, Gassenhuber J, Domdey H, te Heesen S 1996. Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology 6: 439–444 - PubMed
-
- Aebi M, Bernasconi R, Clerc S, Molinari M 2010. N-glycan structures: Recognition and processing in the ER. Trends Biochem Sci 35: 74–82 - PubMed
-
- Allen S, Naim HY, Bulleid NJ 1995. Intracellular folding of tissue-type plasminogen activator. Effects of disulfide bond formation on N-linked glycosylation and secretion. J Biol Chem 270: 4797–4804 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
