Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses
- PMID: 23751205
- PMCID: PMC3773272
- DOI: 10.1016/j.biopsych.2013.04.025
Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses
Abstract
Background: Clinical studies report that scopolamine, an acetylcholine muscarinic receptor antagonist, produces rapid antidepressant effects in depressed patients, but the mechanisms underlying the therapeutic response have not been determined. The present study examines the role of the mammalian target of rapamycin complex 1 (mTORC1) and synaptogenesis, which have been implicated in the rapid actions of N-methyl-D-aspartate receptor antagonists.
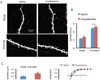
Methods: The influence of scopolamine on mTORC1 signaling was determined by analysis of the phosphorylated and activated forms of mTORC1 signaling proteins in the prefrontal cortex (PFC). The numbers and function of spine synapses were analyzed by whole cell patch clamp recording and two-photon image analysis of PFC neurons. The actions of scopolamine were examined in the forced swim test in the absence or presence of selective mTORC1 and glutamate receptor inhibitors.
Results: The results demonstrate that a single, low dose of scopolamine rapidly increases mTORC1 signaling and the number and function of spine synapses in layer V pyramidal neurons in the PFC. Scopolamine administration also produces an antidepressant response in the forced swim test that is blocked by pretreatment with the mTORC1 inhibitor or by a glutamate alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor antagonist.
Conclusions: Taken together, the results demonstrate that the antidepressant actions of scopolamine require mTORC1 signaling and are associated with increased glutamate transmission, and synaptogenesis, similar to N-methyl-D-aspartate receptor antagonists. These findings provide novel targets for safer and more efficacious rapid-acting antidepressant agents.
Keywords: Acetylcholine; GABA; depression; glutamate; ketamine; synaptic plasticity.
© 2013 Society of Biological Psychiatry.
Conflict of interest statement
Financial Disclosures
The authors reported no biomedical financial interests or potential conflicts of interest.
Figures



References
-
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. - PubMed
-
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. - PubMed
-
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. - PubMed
-
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatmentresistant major depression. Arch Gen Psychiatry. 2006;63:856–864. - PubMed
-
- Liebrenz M, Borgeat A, Leisinger R, Stohler R. Intravenous ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

