Utility of a bacterial infection model to study epithelial-mesenchymal transition, mesenchymal-epithelial transition or tumorigenesis
- PMID: 23752178
- PMCID: PMC3883801
- DOI: 10.1038/onc.2013.210
Utility of a bacterial infection model to study epithelial-mesenchymal transition, mesenchymal-epithelial transition or tumorigenesis
Abstract
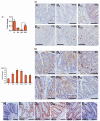
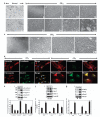
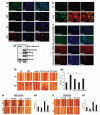
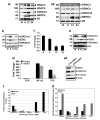
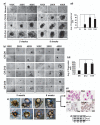
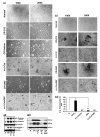
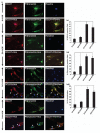
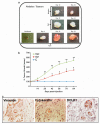
DCLK1 and Lgr5 have recently been identified as markers of quiescent and cycling stem cells in the small intestinal crypts, respectively. Epithelial-mesenchymal transition (EMT) is a key development program that is often activated during cancer invasion and metastasis, and also imparts a self-renewal capability to disseminating cancer cells. Utilizing the Citrobacter rodentium (CR)-induced transmissible murine colonic hyperplasia (TMCH) model, we observed a relative decrease in DCLK1 expression in the colonic crypts, with significant shift towards stromal staining at peak (12 days post infection) hyperplasia, whereas staining for Lgr5 and Msi-1 increased several fold. When hyperplasia was regressing (days 20-34), an expansion of DCLK1+ve cells in the CR-infected crypts compared with that seen in uninfected control was recorded. Purified colonic crypt cells exhibiting epigenetic modulation of the transforming growth factor-β (TGFβ), Wnt and Notch pathways on 12 or 34 days post infection formed monolayers in vitro, and underwent trans-differentiation into fibroblast-like cells that stained positive for vimentin, fibronectin and DCLK1. These cells when trypsinized and regrown in soft agar, formed colonospheres/organoids that developed into crypt-like structures (colonoids) in Matrigel and stained positive for DCLK1. Mice exhibiting 12 or 34 days of TMCH were given azoxymethane once for 8 h (Gp1) or weekly for 3 weeks (Gp2), and subjected to crypt isolation. Crypt cells from Gp1 animals formed monolayers as well as colonospheres in soft agar and nodules/tumors in nude mice. Crypt cells isolated from Gp2 animals failed to form the monolayers, but developed into colonospheres in soft agar and nodules/tumors in nude mice. Thus, both hyperplasia and increased presence of DCLK1+ve cells promote cellular transformation in response to a second hit. The TMCH model, therefore, provides an excellent template to study how alterations in intestinal stem cells promote trans-differentiation, crypt regeneration or colon carcinogenesis following bacterial infection.
Figures

Similar articles
-
Critical roles of Notch and Wnt/β-catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection.Infect Immun. 2012 Sep;80(9):3107-21. doi: 10.1128/IAI.00236-12. Epub 2012 Jun 18. Infect Immun. 2012. PMID: 22710872 Free PMC article.
-
Enteric infection coupled with chronic Notch pathway inhibition alters colonic mucus composition leading to dysbiosis, barrier disruption and colitis.PLoS One. 2018 Nov 1;13(11):e0206701. doi: 10.1371/journal.pone.0206701. eCollection 2018. PLoS One. 2018. PMID: 30383855 Free PMC article.
-
Evidence of functional cross talk between the Notch and NF-κB pathways in nonneoplastic hyperproliferating colonic epithelium.Am J Physiol Gastrointest Liver Physiol. 2013 Feb 15;304(4):G356-70. doi: 10.1152/ajpgi.00372.2012. Epub 2012 Nov 29. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23203159 Free PMC article.
-
Reprogramming during epithelial to mesenchymal transition under the control of TGFβ.Cell Adh Migr. 2015;9(3):233-46. doi: 10.4161/19336918.2014.983794. Epub 2014 Nov 17. Cell Adh Migr. 2015. PMID: 25482613 Free PMC article. Review.
-
Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer.Asian Pac J Cancer Prev. 2015;16(15):6201-6. doi: 10.7314/apjcp.2015.16.15.6201. Asian Pac J Cancer Prev. 2015. PMID: 26434817 Review.
Cited by
-
Interaction between Avian Leukosis Virus Subgroup J Surface Protein and Doublecortin-Like Kinase 1 Accelerates Cell Proliferation and Epithelial-Mesenchymal Transition.J Virol. 2022 Mar 23;96(6):e0165721. doi: 10.1128/JVI.01657-21. Epub 2022 Jan 26. J Virol. 2022. PMID: 35080427 Free PMC article.
-
Bioinformatics and immunohistochemistry analyses of expression levels and clinical significance of CXCL2 and TANs in an oral squamous cell carcinoma tumor microenvironment of Prophyromonas gingivalis infection.Oncol Lett. 2021 Mar;21(3):189. doi: 10.3892/ol.2021.12450. Epub 2021 Jan 6. Oncol Lett. 2021. PMID: 33574928 Free PMC article.
-
Doublecortin-like kinase 1 is elevated serologically in pancreatic ductal adenocarcinoma and widely expressed on circulating tumor cells.PLoS One. 2015 Feb 27;10(2):e0118933. doi: 10.1371/journal.pone.0118933. eCollection 2015. PLoS One. 2015. PMID: 25723399 Free PMC article.
-
Mapping the scientific output of organoids for animal and human modeling infectious diseases: a bibliometric assessment.Vet Res. 2024 Jun 26;55(1):81. doi: 10.1186/s13567-024-01333-7. Vet Res. 2024. PMID: 38926765 Free PMC article.
-
Infection-induced signals generated at the plasma membrane epigenetically regulate Wnt signaling in vitro and in vivo.J Biol Chem. 2020 Jan 24;295(4):1021-1035. doi: 10.1074/jbc.RA119.010285. Epub 2019 Dec 13. J Biol Chem. 2020. PMID: 31836665 Free PMC article.
References
-
- Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8:415–424. - PubMed
-
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Radisky DC, LaBarge MA. Epithelial-mesenchymal transition and the stem cell phenotype. Cell Stem Cell. 2008;2:511–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

