Antifreeze peptides and glycopeptides, and their derivatives: potential uses in biotechnology
- PMID: 23752356
- PMCID: PMC3721219
- DOI: 10.3390/md11062013
Antifreeze peptides and glycopeptides, and their derivatives: potential uses in biotechnology
Abstract
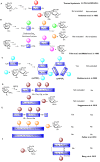
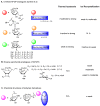
Antifreeze proteins (AFPs) and glycoproteins (AFGPs), collectively called AF(G)Ps, constitute a diverse class of proteins found in various Arctic and Antarctic fish, as well as in amphibians, plants, and insects. These compounds possess the ability to inhibit the formation of ice and are therefore essential to the survival of many marine teleost fishes that routinely encounter sub-zero temperatures. Owing to this property, AF(G)Ps have potential applications in many areas such as storage of cells or tissues at low temperature, ice slurries for refrigeration systems, and food storage. In contrast to AFGPs, which are composed of repeated tripeptide units (Ala-Ala-Thr)n with minor sequence variations, AFPs possess very different primary, secondary, and tertiary structures. The isolation and purification of AFGPs is laborious, costly, and often results in mixtures, making characterization difficult. Recent structural investigations into the mechanism by which linear and cyclic AFGPs inhibit ice crystallization have led to significant progress toward the synthesis and assessment of several synthetic mimics of AFGPs. This review article will summarize synthetic AFGP mimics as well as current challenges in designing compounds capable of mimicking AFGPs. It will also cover our recent efforts in exploring whether peptoid mimics can serve as structural and functional mimics of native AFGPs.
Figures

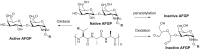



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

