Contributions of environmental signals and conserved residues to the functions of carbon storage regulator A of Borrelia burgdorferi
- PMID: 23753623
- PMCID: PMC3719599
- DOI: 10.1128/IAI.00494-13
Contributions of environmental signals and conserved residues to the functions of carbon storage regulator A of Borrelia burgdorferi
Abstract
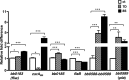
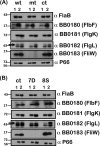
Carbon storage regulator A of Borrelia burgdorferi (CsrABb) contributes to vertebrate host-specific adaptation by modulating activation of the Rrp2-RpoN-RpoS pathway and is critical for infectivity. We hypothesized that the functions of CsrABb are dependent on environmental signals and on select residues. We analyzed the phenotype of csrABb deletion and site-specific mutants to determine the conserved and pathogen-specific attributes of CsrABb. Levels of phosphate acetyltransferase (Pta) involved in conversion of acetyl phosphate to acetyl-coenzyme A (acetyl-CoA) and posttranscriptionally regulated by CsrABb in the csrABb mutant were reduced from or similar to those in the control strains under unfed- or fed-tick conditions, respectively. Increased levels of supplemental acetate restored vertebrate host-responsive determinants in the csrABb mutant to parental levels, indicating that both the levels of CsrABb and the acetyl phosphate and acetyl-CoA balance contribute to the activation of the Rrp2-RpoN-RpoS pathway. Site-specific replacement of 8 key residues of CsrABb (8S) with alanines resulted in increased levels of CsrABb and reduced levels of Pta and acetyl-CoA, while levels of RpoS, BosR, and other members of rpoS regulon were elevated. Truncation of 7 amino acids at the C terminus of CsrABb (7D) resulted in reduced csrABb transcripts and posttranscriptionally reduced levels of FliW located upstream of CsrABb. Electrophoretic mobility shift assays revealed increased binding of 8S mutant protein to the CsrA binding box upstream of pta compared to the parental and 7D truncated protein. Two CsrABb binding sites were also identified upstream of fliW within the flgK coding sequence. These observations reveal conserved and unique functions of CsrABb that regulate adaptive gene expression in B. burgdorferi.
Figures









Similar articles
-
Inactivation of bb0184, which encodes carbon storage regulator A, represses the infectivity of Borrelia burgdorferi.Infect Immun. 2011 Mar;79(3):1270-9. doi: 10.1128/IAI.00871-10. Epub 2010 Dec 20. Infect Immun. 2011. PMID: 21173314 Free PMC article.
-
Overexpression of CsrA (BB0184) alters the morphology and antigen profiles of Borrelia burgdorferi.Infect Immun. 2009 Nov;77(11):5149-62. doi: 10.1128/IAI.00673-09. Epub 2009 Sep 8. Infect Immun. 2009. PMID: 19737901 Free PMC article.
-
Short-Chain Fatty Acids Alter Metabolic and Virulence Attributes of Borrelia burgdorferi.Infect Immun. 2018 Aug 22;86(9):e00217-18. doi: 10.1128/IAI.00217-18. Print 2018 Sep. Infect Immun. 2018. PMID: 29891543 Free PMC article.
-
Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi.PLoS Pathog. 2010 Sep 16;6(9):e1001104. doi: 10.1371/journal.ppat.1001104. PLoS Pathog. 2010. PMID: 20862323 Free PMC article.
-
Gene regulation in Borrelia burgdorferi.Annu Rev Microbiol. 2011;65:479-99. doi: 10.1146/annurev.micro.112408.134040. Annu Rev Microbiol. 2011. PMID: 21801026 Review.
Cited by
-
Borrelia peptidoglycan interacting Protein (BpiP) contributes to the fitness of Borrelia burgdorferi against host-derived factors and influences virulence in mouse models of Lyme disease.PLoS Pathog. 2021 Apr 21;17(4):e1009535. doi: 10.1371/journal.ppat.1009535. eCollection 2021 Apr. PLoS Pathog. 2021. PMID: 33882111 Free PMC article.
-
The Borrelia burgdorferi Glycosaminoglycan Binding Protein Bgp in the B31 Strain Is Not Essential for Infectivity despite Facilitating Adherence and Tissue Colonization.Infect Immun. 2018 Jan 22;86(2):e00667-17. doi: 10.1128/IAI.00667-17. Print 2018 Feb. Infect Immun. 2018. PMID: 29158428 Free PMC article.
-
Role of Dual Specificity Phosphatase 1 (DUSP1) in influencing inflammatory pathways in macrophages modulated by Borrelia burgdorferi lipoproteins.bioRxiv [Preprint]. 2024 Nov 21:2024.11.20.624562. doi: 10.1101/2024.11.20.624562. bioRxiv. 2024. PMID: 39605372 Free PMC article. Preprint.
-
Gene Regulation and Transcriptomics.Curr Issues Mol Biol. 2021;42:223-266. doi: 10.21775/cimb.042.223. Epub 2020 Dec 10. Curr Issues Mol Biol. 2021. PMID: 33300497 Free PMC article.
-
CsrA (BB0184) is not involved in activation of the RpoN-RpoS regulatory pathway in Borrelia burgdorferi.Infect Immun. 2014 Apr;82(4):1511-22. doi: 10.1128/IAI.01555-13. Epub 2014 Jan 22. Infect Immun. 2014. PMID: 24452681 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

