Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis
- PMID: 23754238
- PMCID: PMC4949954
- DOI: 10.1002/art.38044
Clinical outcomes of remission induction therapy for severe antineutrophil cytoplasmic antibody-associated vasculitis
Abstract
Objective: To evaluate the reasons that complete remission is not achieved or maintained with original treatment in some patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) treated with rituximab (RTX) or with cyclophosphamide/azathioprine (CYC/AZA).
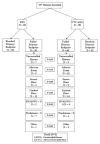
Methods: The Rituximab in AAV trial was a randomized, double-blind, placebo-controlled trial comparing the rate of remission induction among patients treated with RTX (n = 99) and patients treated with CYC followed by AZA (n = 98). Glucocorticoids were tapered over a period of 5 months. The primary outcome measure was lack of disease activity without glucocorticoid treatment at 6 months. To determine the most important reason for failure to achieve the primary outcome, 7 hierarchical categories of reasons were defined retrospectively (uncontrolled disease, adverse event leading to therapy discontinuation, severe flare, limited flare, Birmingham Vasculitis Activity Score for Wegener's Granulomatosis >0, prednisone treatment at any dosage, and other).
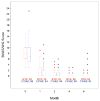
Results: Although remission (lack of disease activity) was achieved in 170 of the 197 patients (86%) in the first 6 months, the primary outcome measure was not achieved in 42%. There were 3 deaths. Twenty-four percent of the patients failed to achieve the primary end point due to active disease: 10 (5%) experienced uncontrolled disease in the first month and 37 (19%) experienced flares after initial improvement. In the majority of such patients, treatment with blinded crossover or according to best medical judgment led to disease control. Ninety-one percent of patients who had uncontrolled disease or experienced a severe flare had proteinase 3 (PR3)-ANCA. When patients with uncontrolled disease were excluded from analysis, those who were PR3-ANCA positive were found to experience fewer flares when treated with RTX compared to CYC/AZA (8 of 59 [14%] versus 20 of 62 [32%]; P = 0.02). Neither ANCA titers nor B cell counts predicted disease flare.
Conclusion: Current treatment regimens are largely successful in controlling AAV, but in approximately one-fourth of patients, active disease persists or recurs in the first 6 months despite treatment. PR3-ANCA positivity is a risk factor for recurrence or persistence of severe disease. ANCA titers and B cell detectability are poor predictors of both disease relapse and disease quiescence in the first 6 months.
Trial registration: ClinicalTrials.gov NCT00104299.
Copyright © 2013 by the American College of Rheumatology.
Figures
References
-
- Fauci AS, Katz P, Haynes BF, Wolff SM. Cyclophosphamide therapy of severe systemic necrotizing vasculitis. N Engl J Med. 1979;301:235–8. - PubMed
-
- Hoffman GS, Kerr GS, Leavitt RY, Hallahan CW, Lebovics RS, Travis WD, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med. 1992;116:488–98. - PubMed
-
- Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. for the International Network for the Study of the Systemic Vasculitides (INSSYS) A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. - PubMed
-
- Specks U, Merkel PA, Hoffman GS, Langford CA, Spiera R, Seo P, et al. for the RAVE-ITN Research Group. Design of the Rituximab in ANCA-associated Vasculitis (RAVE) Trial. Open Arthritis J. 2011;4:1–18.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- RR-024150-01/RR/NCRR NIH HHS/United States
- RR-025771/RR/NCRR NIH HHS/United States
- UL1 RR025005/RR/NCRR NIH HHS/United States
- N01 AI015416/AI/NIAID NIH HHS/United States
- K23 AR052820/AR/NIAMS NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- K24 AR002224/AR/NIAMS NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- K24 AR049185/AR/NIAMS NIH HHS/United States
- U01 AI067075/AI/NIAID NIH HHS/United States
- UL1 RR024150/RR/NCRR NIH HHS/United States
- K23-AR-052820/AR/NIAMS NIH HHS/United States
- U01 AI088635/AI/NIAID NIH HHS/United States
- RR-025005/RR/NCRR NIH HHS/United States
- M01-RR-00533/RR/NCRR NIH HHS/United States
- K24-AR-049185/AR/NIAMS NIH HHS/United States
- K24-AR-02224/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

