Strain history dependence of the nonlinear stress response of fibrin and collagen networks
- PMID: 23754380
- PMCID: PMC3725119
- DOI: 10.1073/pnas.1222787110
Strain history dependence of the nonlinear stress response of fibrin and collagen networks
Abstract
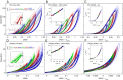
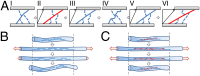
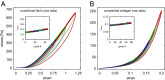
We show that the nonlinear mechanical response of networks formed from un-cross-linked fibrin or collagen type I continually changes in response to repeated large-strain loading. We demonstrate that this dynamic evolution of the mechanical response arises from a shift of a characteristic nonlinear stress-strain relationship to higher strains. Therefore, the imposed loading does not weaken the underlying matrices but instead delays the occurrence of the strain stiffening. Using confocal microscopy, we present direct evidence that this behavior results from persistent lengthening of individual fibers caused by an interplay between fiber stretching and fiber buckling when the networks are repeatedly strained. Moreover, we show that covalent cross-linking of fibrin or collagen inhibits the shift of the nonlinear material response, suggesting that the molecular origin of individual fiber lengthening may be slip of monomers within the fibers. Thus, a fibrous architecture in combination with constituents that exhibit internal plasticity creates a material whose mechanical response adapts to external loading conditions. This design principle may be useful to engineer novel materials with this capability.
Keywords: ECM; blood clot; factor XIII; nonlinear rheology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Comment in
-
Adaptation of fibrous biopolymers to recurring increasing strains.Proc Natl Acad Sci U S A. 2013 Jul 23;110(30):12164-5. doi: 10.1073/pnas.1310351110. Epub 2013 Jul 10. Proc Natl Acad Sci U S A. 2013. PMID: 23842087 Free PMC article. No abstract available.
References
-
- Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophys Chem. 2004;112(2–3):267–276. - PubMed
-
- Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55(12):1531–1546. - PubMed
-
- Janmey PA, Amis EJ, Ferry JD. Rheology of fibrin clots 6. Stress-relaxation, creep, and differential dynamic modulus of fine clots in large shearing deformations. J Rheol (N Y N Y) 1983;27(2):135–153.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

