Posttranscriptional regulation of retroviral gene expression: primary RNA transcripts play three roles as pre-mRNA, mRNA, and genomic RNA
- PMID: 23754689
- PMCID: PMC3810403
- DOI: 10.1002/wrna.1179
Posttranscriptional regulation of retroviral gene expression: primary RNA transcripts play three roles as pre-mRNA, mRNA, and genomic RNA
Abstract
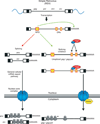
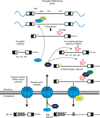
After reverse transcription of the retroviral RNA genome and integration of the DNA provirus into the host genome, host machinery is used for viral gene expression along with viral proteins and RNA regulatory elements. Here, we discuss co-transcriptional and posttranscriptional regulation of retroviral gene expression, comparing simple and complex retroviruses. Cellular RNA polymerase II synthesizes full-length viral primary RNA transcripts that are capped and polyadenylated. All retroviruses generate a singly spliced env mRNA from this primary transcript, which encodes the viral glycoproteins. In addition, complex viral RNAs are alternatively spliced to generate accessory proteins, such as Rev, which is involved in posttranscriptional regulation of HIV-1 RNA. Importantly, the splicing of all retroviruses is incomplete; they must maintain and export a fraction of their primary RNA transcripts. This unspliced RNA functions both as the major mRNA for Gag and Pol proteins and as the packaged genomic RNA. Different retroviruses export their unspliced viral RNA from the nucleus to the cytoplasm by either Tap-dependent or Rev/CRM1-dependent routes. Translation of the unspliced mRNA involves frame-shifting or termination codon suppression so that the Gag proteins, which make up the capsid, are expressed more abundantly than the Pol proteins, which are the viral enzymes. After the viral polyproteins assemble into viral particles and bud from the cell membrane, a viral encoded protease cleaves them. Some retroviruses have evolved mechanisms to protect their unspliced RNA from decay by nonsense-mediated RNA decay and to prevent genome editing by the cellular APOBEC deaminases.
Copyright © 2013 John Wiley & Sons, Ltd.
Conflict of interest statement
Conflict of interest: The authors have declared no conflicts of interest for this article.
Figures
References
-
- Coffin JM, Hughes SH, Varmus HE. Retroviruses. New York: Cold Spring Harbor Laboratory Press; 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

