The inevitable journey to being
- PMID: 23754808
- PMCID: PMC3685457
- DOI: 10.1098/rstb.2012.0254
The inevitable journey to being
Abstract
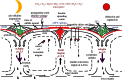
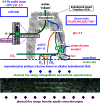
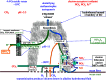
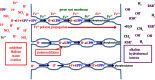
Life is evolutionarily the most complex of the emergent symmetry-breaking, macroscopically organized dynamic structures in the Universe. Members of this cascading series of disequilibria-converting systems, or engines in Cottrell's terminology, become ever more complicated-more chemical and less physical-as each engine extracts, exploits and generates ever lower grades of energy and resources in the service of entropy generation. Each one of these engines emerges spontaneously from order created by a particular mother engine or engines, as the disequilibrated potential daughter is driven beyond a critical point. Exothermic serpentinization of ocean crust is life's mother engine. It drives alkaline hydrothermal convection and thereby the spontaneous production of precipitated submarine hydrothermal mounds. Here, the two chemical disequilibria directly causative in the emergence of life spontaneously arose across the mineral precipitate membranes separating the acidulous, nitrate-bearing CO2-rich, Hadean sea from the alkaline and CH4/H2-rich serpentinization-generated effluents. Essential redox gradients-involving hydrothermal CH4 and H2 as electron donors, CO2 and nitrate, nitrite, and ferric iron from the ambient ocean as acceptors-were imposed which functioned as the original 'carbon-fixing engine'. At the same time, a post-critical-point (milli)voltage pH potential (proton concentration gradient) drove the condensation of orthophosphate to produce a high energy currency: 'the pyrophosphatase engine'.
Keywords: alkaline hydrothermal; carbon fixation; disequilibria; origin of life; pyrophosphatase.
Figures

Similar articles
-
A self-sustaining serpentinization mega-engine feeds the fougerite nanoengines implicated in the emergence of guided metabolism.Front Microbiol. 2023 May 15;14:1145915. doi: 10.3389/fmicb.2023.1145915. eCollection 2023. Front Microbiol. 2023. PMID: 37275164 Free PMC article.
-
The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front.J Geol Soc London. 1997 May;154(3):377-402. doi: 10.1144/gsjgs.154.3.0377. J Geol Soc London. 1997. PMID: 11541234
-
Simulating Serpentinization as It Could Apply to the Emergence of Life Using the JPL Hydrothermal Reactor.Astrobiology. 2020 Mar;20(3):307-326. doi: 10.1089/ast.2018.1949. Astrobiology. 2020. PMID: 32125196
-
Green Rust: The Simple Organizing 'Seed' of All Life?Life (Basel). 2018 Aug 27;8(3):35. doi: 10.3390/life8030035. Life (Basel). 2018. PMID: 30150570 Free PMC article. Review.
-
Natural pH Gradients in Hydrothermal Alkali Vents Were Unlikely to Have Played a Role in the Origin of Life.J Mol Evol. 2016 Aug;83(1-2):1-11. doi: 10.1007/s00239-016-9756-6. Epub 2016 Aug 17. J Mol Evol. 2016. PMID: 27534947 Free PMC article. Review.
Cited by
-
Energy, genes and evolution: introduction to an evolutionary synthesis.Philos Trans R Soc Lond B Biol Sci. 2013 Jun 10;368(1622):20120253. doi: 10.1098/rstb.2012.0253. Print 2013 Jul 19. Philos Trans R Soc Lond B Biol Sci. 2013. PMID: 23754807 Free PMC article.
-
The Cosmic Zoo: The (Near) Inevitability of the Evolution of Complex, Macroscopic Life.Life (Basel). 2016 Jun 30;6(3):25. doi: 10.3390/life6030025. Life (Basel). 2016. PMID: 27376334 Free PMC article.
-
Fougerite: the not so simple progenitor of the first cells.Interface Focus. 2019 Dec 6;9(6):20190063. doi: 10.1098/rsfs.2019.0063. Epub 2019 Oct 18. Interface Focus. 2019. PMID: 31641434 Free PMC article. Review.
-
Methane: Fuel or Exhaust at the Emergence of Life?Astrobiology. 2017 Oct;17(10):1053-1066. doi: 10.1089/ast.2016.1599. Epub 2017 Sep 26. Astrobiology. 2017. PMID: 28949766 Free PMC article.
-
On the beneficent thickness of water.Interface Focus. 2019 Dec 6;9(6):20190061. doi: 10.1098/rsfs.2019.0061. Epub 2019 Oct 18. Interface Focus. 2019. PMID: 31641433 Free PMC article. Review.
References
-
- Boltzmann L. 1886. The second law of thermodynamics In Ludwig Boltzmann: theoretical physics and philosophical problems: selected writings (Vienna circle collection) (ed. McGuinness BF.). Dordrecht, The Netherlands: Reidel (reprint)
-
- Lane N, Allen JF, Martin W. 2010. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32, 271–28010.1002/bies.200900131 (doi:10.1002/bies.200900131) - DOI - DOI - PubMed
-
- Wood BJ, Bryndzia LT, Johnson KE. 1990. Mantle oxidation state and its relation to tectonic environment and fluid speciation. Science 248, 337–34510.1126/science.248.4953.337 (doi:10.1126/science.248.4953.337) - DOI - DOI - PubMed
-
- Schrödinger E. 1944. What is life? The physical aspects of the living cell. Cambridge, UK: Cambridge University Press
-
- Harris H. 1999. The birth of the cell. New Haven, CT: Yale University Press
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

