The energetics of organic synthesis inside and outside the cell
- PMID: 23754809
- PMCID: PMC3685458
- DOI: 10.1098/rstb.2012.0255
The energetics of organic synthesis inside and outside the cell
Abstract
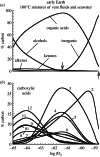
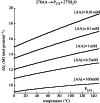
Thermodynamic modelling of organic synthesis has largely been focused on deep-sea hydrothermal systems. When seawater mixes with hydrothermal fluids, redox gradients are established that serve as potential energy sources for the formation of organic compounds and biomolecules from inorganic starting materials. This energetic drive, which varies substantially depending on the type of host rock, is present and available both for abiotic (outside the cell) and biotic (inside the cell) processes. Here, we review and interpret a library of theoretical studies that target organic synthesis energetics. The biogeochemical scenarios evaluated include those in present-day hydrothermal systems and in putative early Earth environments. It is consistently and repeatedly shown in these studies that the formation of relatively simple organic compounds and biomolecules can be energy-yielding (exergonic) at conditions that occur in hydrothermal systems. Expanding on our ability to calculate biomass synthesis energetics, we also present here a new approach for estimating the energetics of polymerization reactions, specifically those associated with polypeptide formation from the requisite amino acids.
Keywords: bioenergetics; biomolecule synthesis; hydrothermal systems; organic synthesis; polypeptide formation.
Figures




References
-
- Martin W, Baross JA, Kelley DS, Russell MJ. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–81410.1038/nrmicro1991 (doi:10.1038/nrmicro1991) - DOI - DOI - PubMed
-
- Schwartzman DW, Lineweaver CH. 2004. The hyperthermophilic origin of life revisited. Biochem. Soc. Trans. 32, 168–17110.1042/BST0320168 (doi:10.1042/BST0320168) - DOI - DOI - PubMed
-
- Pace NR. 1991. Origin of life: facing up to the physical setting. Cell 65, 531–53310.1016/0092-8674(91)90082-A (doi:10.1016/0092-8674(91)90082-A) - DOI - DOI - PubMed
-
- Cody GD, Boctor NZ, Filley TR, Hazen RM, Scott JH, Sharma A, Yoder HS., Jr 2000. Primordial carbonylated iron–sulfur compounds and the synthesis of pyruvate. Science 289, 1337–134010.1126/science.289.5483.1337 (doi:10.1126/science.289.5483.1337) - DOI - DOI - PubMed
-
- McCollom TM, Seewald JS. 2007. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107, 382–40110.1021/cr0503660 (doi:10.1021/cr0503660) - DOI - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

