Energy, ecology and the distribution of microbial life
- PMID: 23754819
- PMCID: PMC3685468
- DOI: 10.1098/rstb.2012.0383
Energy, ecology and the distribution of microbial life
Abstract
Mechanisms that govern the coexistence of multiple biological species have been studied intensively by ecologists since the turn of the nineteenth century. Microbial ecologists in the meantime have faced many fundamental challenges, such as the lack of an ecologically coherent species definition, lack of adequate methods for evaluating population sizes and community composition in nature, and enormous taxonomic and functional diversity. The accessibility of powerful, culture-independent molecular microbiology methods offers an opportunity to close the gap between microbial science and the main stream of ecological theory, with the promise of new insights and tools needed to meet the grand challenges humans face as planetary engineers and galactic explorers. We focus specifically on resources related to energy metabolism because of their direct links to elemental cycling in the Earth's history, engineering applications and astrobiology. To what extent does the availability of energy resources structure microbial communities in nature? Our recent work on sulfur- and iron-oxidizing autotrophs suggests that apparently subtle variations in the concentration ratios of external electron donors and acceptors select for different microbial populations. We show that quantitative knowledge of microbial energy niches (population-specific patterns of energy resource use) can be used to predict variations in the abundance of specific taxa in microbial communities. Furthermore, we propose that resource ratio theory applied to micro-organisms will provide a useful framework for identifying how environmental communities are organized in space and time.
Keywords: biogeochemistry; biogeography; ecological niche; environmental engineering; evolution; resource ratio theory.
Figures
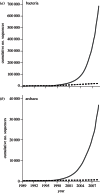




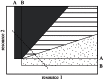
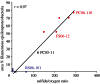

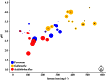
References
-
- Carlson R. 2009. The new biofactories in what matters. McKinsey Quarterly New York, NY: McKinsey and Company; See http://whatmatters.mckinseydigital.com/biotechnology/the-new-biofactories.
-
- Pace NR. 2009. Mapping the tree of life: progress and prospects. Mol. Microbiol. Rev. 73, 565–576 10.1128/MMBR.00033-09 (doi:10.1128/MMBR.00033-09) - DOI - PMC - PubMed
-
- Madsen EL. 2008. Environmental microbiology: from genomes to biogeochemistry. Malden, MA: Blackwell Publishing
-
- Hugenholtz P. 2002. Exploring prokaryotic diversity in the genomic era. Genome Biol. 3, 1–8 10.1186/gb-2002-3-2-reviews0003 (doi:10.1186/gb-2002-3-2-reviews0003) - DOI - PMC - PubMed
-
- Rappe MS, Giovannoni SJ. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57, 369–394 10.1146/annurev.micro.57.030502.090759 (doi:10.1146/annurev.micro.57.030502.090759) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
