Early bioenergetic evolution
- PMID: 23754820
- PMCID: PMC3685469
- DOI: 10.1098/rstb.2013.0088
Early bioenergetic evolution
Abstract
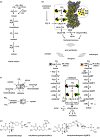
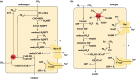
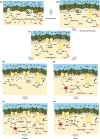
Life is the harnessing of chemical energy in such a way that the energy-harnessing device makes a copy of itself. This paper outlines an energetically feasible path from a particular inorganic setting for the origin of life to the first free-living cells. The sources of energy available to early organic synthesis, early evolving systems and early cells stand in the foreground, as do the possible mechanisms of their conversion into harnessable chemical energy for synthetic reactions. With regard to the possible temporal sequence of events, we focus on: (i) alkaline hydrothermal vents as the far-from-equilibrium setting, (ii) the Wood-Ljungdahl (acetyl-CoA) pathway as the route that could have underpinned carbon assimilation for these processes, (iii) biochemical divergence, within the naturally formed inorganic compartments at a hydrothermal mound, of geochemically confined replicating entities with a complexity below that of free-living prokaryotes, and (iv) acetogenesis and methanogenesis as the ancestral forms of carbon and energy metabolism in the first free-living ancestors of the eubacteria and archaebacteria, respectively. In terms of the main evolutionary transitions in early bioenergetic evolution, we focus on: (i) thioester-dependent substrate-level phosphorylations, (ii) harnessing of naturally existing proton gradients at the vent-ocean interface via the ATP synthase, (iii) harnessing of Na(+) gradients generated by H(+)/Na(+) antiporters, (iv) flavin-based bifurcation-dependent gradient generation, and finally (v) quinone-based (and Q-cycle-dependent) proton gradient generation. Of those five transitions, the first four are posited to have taken place at the vent. Ultimately, all of these bioenergetic processes depend, even today, upon CO2 reduction with low-potential ferredoxin (Fd), generated either chemosynthetically or photosynthetically, suggesting a reaction of the type 'reduced iron → reduced carbon' at the beginning of bioenergetic evolution.
Keywords: acetogens; hydrothermal vents; methanogens; origin of life; sulfate reducers; transition metals.
Figures




References
-
- Stüeken EE, Anderson RE, Bowman JS, Brazelton WJ, Colangelo-Lillis J, Goldman AD, Som SM, Baross JA. 2013. Did life originate from a global chemical reactor? Geobiology 11, 101–126 10.1111/gbi.12025 (doi:10.1111/gbi.12025) - DOI - PubMed
-
- Haldane JBS. 1929. The origin of life. Rationalist Ann. 3, 3–10
-
- de Duve C. 1994. Vital dust: life as a cosmic imperative. New York, NY: Harper Collins
-
- Ricardo A, Carrigan MA, Olcott AN, Benner SA. 2004. Borate minerals stabilize ribose. Science 303, 196. 10.1126/science.1092464 (doi:10.1126/science.1092464) - DOI - PubMed
-
- Mulkidjanian AY, Bychkov AY, Dibrova DV, Galperin MY, Koonin EV. 2012. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl Acad. Sci. USA 109, E821–E830 10.1073/pnas.1117774109 (doi:10.1073/pnas.1117774109) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
