Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans
- PMID: 23754960
- PMCID: PMC3675029
- DOI: 10.1371/journal.pgen.1003536
Mutations in CERS3 cause autosomal recessive congenital ichthyosis in humans
Erratum in
- PLoS Genet. 2013 Jun;9(6): doi/10.1371/annotation/df5af830-8e1d-495a-a206-f881ed85e7fe
Abstract
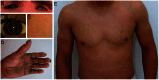
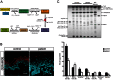
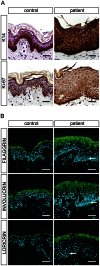
Autosomal recessive congenital ichthyosis (ARCI) is a rare genetic disorder of the skin characterized by abnormal desquamation over the whole body. In this study we report four patients from three consanguineous Tunisian families with skin, eye, heart, and skeletal anomalies, who harbor a homozygous contiguous gene deletion syndrome on chromosome 15q26.3. Genome-wide SNP-genotyping revealed a homozygous region in all affected individuals, including the same microdeletion that partially affects two coding genes (ADAMTS17, CERS3) and abolishes a sequence for a long non-coding RNA (FLJ42289). Whereas mutations in ADAMTS17 have recently been identified in autosomal recessive Weill-Marchesani-like syndrome in humans and dogs presenting with ophthalmologic, cardiac, and skeletal abnormalities, no disease associations have been described for CERS3 (ceramide synthase 3) and FLJ42289 so far. However, analysis of additional patients with non-syndromic ARCI revealed a splice site mutation in CERS3 indicating that a defect in ceramide synthesis is causative for the present skin phenotype of our patients. Functional analysis of patient skin and in vitro differentiated keratinocytes demonstrated that mutations in CERS3 lead to a disturbed sphingolipid profile with reduced levels of epidermis-specific very long-chain ceramides that interferes with epidermal differentiation. Taken together, these data present a novel pathway involved in ARCI development and, moreover, provide the first evidence that CERS3 plays an essential role in human sphingolipid metabolism for the maintenance of epidermal lipid homeostasis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Lefévre C, Audebert S, Jobard F, Bouadjar B, Lakhdar H, et al. (2003) Mutations in the transporter ABCA12 are associated with lamellar ichthyosis type 2. Hum Mol Genet 12: 2369–2378. - PubMed
-
- Jobard F, Lefèvre C, Karaduman A, Blanchet-Bardon C, Emre S, et al. (2002) Lipoxygenase-3 (ALOXE3) and 12(R)-lipoxygenase (ALOX12B) are mutated in non-bullous congenital ichthyosiform erythroderma (NCIE) linked to chromosome 17p13.1. Hum Mol Genet 11: 107–113. - PubMed
-
- Lefèvre C, Bouadjar B, Ferrand V, Tadini G, Mégarbané A, et al. (2006) Mutations in a new cytochrome P450 gene in lamellar ichthyosis type 3. Hum Mol Genet 15: 767–776. - PubMed
-
- Lefèvre C, Bouadjar B, Karaduman A, Jobard F, Saker S, et al. (2004) Mutations in ichthyin a new gene on chromosome 5q33 in a new form of autosomal recessive congenital ichthyosis. Hum Mol Genet 13: 2473–2482. - PubMed
-
- Grall A, Guaguère E, Planchais S, Grond S, Bourrat E, et al. (2012) PNPLA1 mutations cause autosomal recessive congenital ichthyosis in golden retriever dogs and humans. Nat Genet 44: 140–147 doi:10.1038/ng.1056 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

