Negative regulation of notch signaling by xylose
- PMID: 23754965
- PMCID: PMC3675014
- DOI: 10.1371/journal.pgen.1003547
Negative regulation of notch signaling by xylose
Abstract
The Notch signaling pathway controls a large number of processes during animal development and adult homeostasis. One of the conserved post-translational modifications of the Notch receptors is the addition of an O-linked glucose to epidermal growth factor-like (EGF) repeats with a C-X-S-X-(P/A)-C motif by Protein O-glucosyltransferase 1 (POGLUT1; Rumi in Drosophila). Genetic experiments in flies and mice, and in vivo structure-function analysis in flies indicate that O-glucose residues promote Notch signaling. The O-glucose residues on mammalian Notch1 and Notch2 proteins are efficiently extended by the addition of one or two xylose residues through the function of specific mammalian xylosyltransferases. However, the contribution of xylosylation to Notch signaling is not known. Here, we identify the Drosophila enzyme Shams responsible for the addition of xylose to O-glucose on EGF repeats. Surprisingly, loss- and gain-of-function experiments strongly suggest that xylose negatively regulates Notch signaling, opposite to the role played by glucose residues. Mass spectrometric analysis of Drosophila Notch indicates that addition of xylose to O-glucosylated Notch EGF repeats is limited to EGF14-20. A Notch transgene with mutations in the O-glucosylation sites of Notch EGF16-20 recapitulates the shams loss-of-function phenotypes, and suppresses the phenotypes caused by the overexpression of human xylosyltransferases. Antibody staining in animals with decreased Notch xylosylation indicates that xylose residues on EGF16-20 negatively regulate the surface expression of the Notch receptor. Our studies uncover a specific role for xylose in the regulation of the Drosophila Notch signaling, and suggest a previously unrecognized regulatory role for EGF16-20 of Notch.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


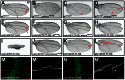

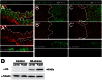
References
-
- Artavanis-Tsakonas S, Muskavitch MA (2010) Notch: the past, the present, and the future. Curr Top Dev Biol 92: 1–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

