Glucose and lactate metabolism in the awake and stimulated rat: a (13)C-NMR study
- PMID: 23755012
- PMCID: PMC3668265
- DOI: 10.3389/fnene.2013.00005
Glucose and lactate metabolism in the awake and stimulated rat: a (13)C-NMR study
Abstract
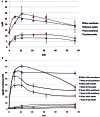
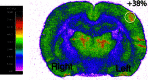
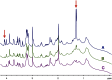
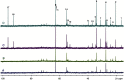
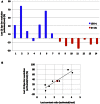
Glucose is the major energetic substrate for the brain but evidence has accumulated during the last 20 years that lactate produced by astrocytes could be an additional substrate for neurons. However, little information exists about this lactate shuttle in vivo in activated and awake animals. We designed an experiment in which the cortical barrel field (S1BF) was unilaterally activated during infusion of both glucose and lactate (alternatively labeled with (13)C) in rats. At the end of stimulation (1 h) both S1BF areas were removed and analyzed by HR-MAS NMR spectroscopy to compare glucose and lactate metabolism in the activated area vs. the non-activated one. In combination with microwave irradiation HR-MAS spectroscopy is a powerful technical approach to study brain lactate metabolism in vivo. Using in vivo (14)C-2-deoxyglucose and autoradiography we confirmed that whisker stimulation was effective since we observed a 40% increase in glucose uptake in the activated S1BF area compared to the ipsilateral one. We first determined that lactate observed on spectra of biopsies did not arise from post-mortem metabolism. (1)H-NMR data indicated that during brain activation there was an average 2.4-fold increase in lactate content in the activated area. When [1-(13)C]glucose + lactate were infused (13)C-NMR data showed an increase in (13)C-labeled lactate during brain activation as well as an increase in lactate C3-specific enrichment. This result demonstrates that the increase in lactate observed on (1)H-NMR spectra originates from newly synthesized lactate from the labeled precursor ([1-(13)C]glucose). It also shows that this additional lactate does not arise from an increase in blood lactate uptake since it would otherwise be unlabeled. These results are in favor of intracerebral lactate production during brain activation in vivo which could be a supplementary fuel for neurons.
Keywords: 13C NMR spectroscopy; astrocytes; brain metabolism; lactate; neurons.
Figures

References
-
- Abi-Saab W. M., Maggs D. G., Jones T., Jacob R., Srihari V., Thompson J., et al. (2002). Striking differences in glucose and lactate levels between brain extracellular fluid and plasma in conscious human subjects: effects of hyperglycemia and hypoglycemia. J. Cereb. Blood Flow Metab. 22, 271–279 10.1097/00004647-200203000-00004 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

