Development and evaluation of a pseudovirus-luciferase assay for rapid and quantitative detection of neutralizing antibodies against enterovirus 71
- PMID: 23755115
- PMCID: PMC3673970
- DOI: 10.1371/journal.pone.0064116
Development and evaluation of a pseudovirus-luciferase assay for rapid and quantitative detection of neutralizing antibodies against enterovirus 71
Abstract
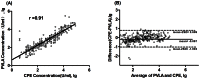
The level of neutralizing antibodies (NtAb) induced by vaccine inoculation is an important endpoint to evaluate the efficacy of EV71 vaccine. In order to evaluate the efficacy of EV71 vaccine, here, we reported the development of a novel pseudovirus system expression firefly luciferase (PVLA) for the quantitative measurement of NtAb. We first evaluated and validated the sensitivity and specificity of the PVLA method. A total of 326 serum samples from an epidemiological survey and 144 serum specimens from 3 clinical trials of EV71 vaccines were used, and the level of each specimen's neutralizing antibodies (NtAb) was measured in parallel using both the conventional CPE-based and PVLA-based assay. Against the standard neutralization assay based on the inhibition of the cytopathic effect (CPE), the sensitivity and specificity of the PVLA method are 98% and 96%, respectively. Then, we tested the potential interference of NtAb against hepatitis A virus, Polio-I, Polio-II, and Polio-III standard antisera (WHO) and goat anti-G10/CA16 serum, the PVLA based assay showed no cross-reactivity with NtAb against other specific sera. Importantly, unlike CPE based method, no live replication-competent EV71 is used during the measurement. Taken together, PVLA is a rapid and specific assay with higher sensitivity and accuracy. It could serve as a valuable tool in assessing the efficacy of EV71 vaccines in clinical trials and disease surveillance in epidemiology studies.
Conflict of interest statement
Figures


References
-
- Hsu CH, Lu CY, Shao PL, Lee PI, Kao CL, et al. (2008) Epidemiologic and clinical features of non-polio enteroviral infections in northern Taiwan in 2008. J Microbiol Immunol Infect 44: 265–273. - PubMed
-
- Chang LY, Lin TY, Huang YC, Tsao KC, Shih SR, et al. (1999) Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J 18: 1092–1096. - PubMed
-
- Schmidt NJ, Lennette EH, Ho HH (1974) An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129: 304–309. - PubMed
-
- Chang LY (2008) Enterovirus 71 in Taiwan. Pediatr Neonatol 49: 103–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

