Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue
- PMID: 23755141
- PMCID: PMC3673968
- DOI: 10.1371/journal.pone.0064752
Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue
Erratum in
- PLoS One. 2013;8(7). doi:10.1371/annotation/190d4d01-a63c-4adc-a123-e519ee40a03e
Abstract
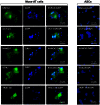
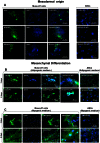
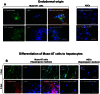
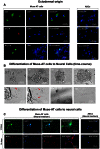
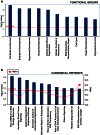
Advances in stem cell therapy face major clinical limitations, particularly challenged by low rates of post-transplant cell survival. Hostile host factors of the engraftment microenvironment such as hypoxia, nutrition deprivation, pro-inflammatory cytokines, and reactive oxygen species can each contribute to unwanted differentiation or apoptosis. In this report, we describe the isolation and characterization of a new population of adipose tissue (AT) derived pluripotent stem cells, termed Multilineage Differentiating Stress-Enduring (Muse) Cells, which are isolated using severe cellular stress conditions, including long-term exposure to the proteolytic enzyme collagenase, serum deprivation, low temperatures and hypoxia. Under these conditions, a highly purified population of Muse-AT cells is isolated without the utilization of cell sorting methods. Muse-AT cells grow in suspension as cell spheres reminiscent of embryonic stem cell clusters. Muse-AT cells are positive for the pluripotency markers SSEA3, TR-1-60, Oct3/4, Nanog and Sox2, and can spontaneously differentiate into mesenchymal, endodermal and ectodermal cell lineages with an efficiency of 23%, 20% and 22%, respectively. When using specific differentiation media, differentiation efficiency is greatly enhanced in Muse-AT cells (82% for mesenchymal, 75% for endodermal and 78% for ectodermal). When compared to adipose stem cells (ASCs), microarray data indicate a substantial up-regulation of Sox2, Oct3/4, and Rex1. Muse-ATs also exhibit gene expression patterns associated with the down-regulation of genes involved in cell death and survival, embryonic development, DNA replication and repair, cell cycle and potential factors related to oncogenecity. Gene expression analysis indicates that Muse-ATs and ASCs are mesenchymal in origin; however, Muse-ATs also express numerous lymphocytic and hematopoietic genes, such as CCR1 and CXCL2, encoding chemokine receptors and ligands involved in stem cell homing. Being highly resistant to severe cellular stress, Muse-AT cells have the potential to make a critical impact on the field of regenerative medicine and cell-based therapy.
Conflict of interest statement
Figures

References
-
- Hodgetts SI, Beilharz MW, Scalzo AA, Grounds MD (2000) Why do cultured transplanted myoblasts die in vivo? DNA quantification shows enhanced survival of donor male myoblasts in host mice depleted of CD4+ and CD8+ cells or Nk1.1+ cells. Cell Transplant 9: 489–502. - PubMed
-
- Oh JS, Kim KN, An SS, Pennant WA, Kim HJ, et al. (2010) Cotransplantation of mouse neural stem cells (mNSCs) with adipose tissue-derived mesenchymal stem cells improves mNSC survival in a rat spinal cord injury model. Cell Transplant 20: 837–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

