Exonuclease hDIS3L2 specifies an exosome-independent 3'-5' degradation pathway of human cytoplasmic mRNA
- PMID: 23756462
- PMCID: PMC3981170
- DOI: 10.1038/emboj.2013.135
Exonuclease hDIS3L2 specifies an exosome-independent 3'-5' degradation pathway of human cytoplasmic mRNA
Abstract
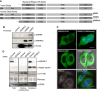
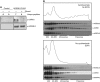
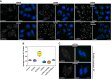
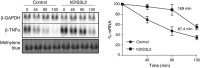
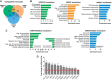
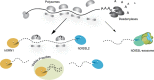
Turnover of mRNA in the cytoplasm of human cells is thought to be redundantly conducted by the monomeric 5'-3' exoribonuclease hXRN1 and the 3'-5' exoribonucleolytic RNA exosome complex. However, in addition to the exosome-associated 3'-5' exonucleases hDIS3 and hDIS3L, the human genome encodes another RNase II/R domain protein-hDIS3L2. Here, we show that hDIS3L2 is an exosome-independent cytoplasmic mRNA 3'-5' exonuclease, which exhibits processive activity on structured RNA substrates in vitro. hDIS3L2 associates with hXRN1 in an RNA-dependent manner and can, like hXRN1, be found on polysomes. The impact of hDIS3L2 on cytoplasmic RNA metabolism is revealed by an increase in levels of cytoplasmic RNA processing bodies (P-bodies) upon hDIS3L2 depletion, which also increases half-lives of investigated mRNAs. Consistently, RNA sequencing (RNA-seq) analyses demonstrate that depletion of hDIS3L2, like downregulation of hXRN1 and hDIS3L, causes changed levels of multiple mRNAs. We suggest that hDIS3L2 is a key exosome-independent effector of cytoplasmic mRNA metabolism.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Comment in
-
A DIStinctively novel exoribonuclease that really likes U.EMBO J. 2013 Jul 3;32(13):1799-801. doi: 10.1038/emboj.2013.136. Epub 2013 Jun 11. EMBO J. 2013. PMID: 23756464 Free PMC article.
References
-
- Allalou A, Wahlby C (2009) BlobFinder, a tool for fluorescence microscopy image cytometry. Comput Methods Prog Biomed 94: 58–65 - PubMed
-
- Astuti D, Morris MR, Cooper WN, Staals RH, Wake NC, Fews GA, Gill H, Gentle D, Shuib S, Ricketts CJ, Cole T, van Essen AJ, van Lingen RA, Neri G, Opitz JM, Rump P, Stolte-Dijkstra I, Muller F, Pruijn GJ, Latif F et al. (2012) Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44: 277–284 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

