Mechanosensitive ATP release maintains proper mucus hydration of airways
- PMID: 23757023
- PMCID: PMC3791865
- DOI: 10.1126/scisignal.2003755
Mechanosensitive ATP release maintains proper mucus hydration of airways
Abstract
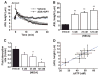
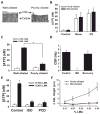
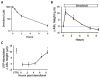
The clearance of mucus from the airways protects the lungs from inhaled noxious and infectious materials. Proper hydration of the mucus layer enables efficient mucus clearance through beating of cilia on airway epithelial cells, and reduced clearance of excessively concentrated mucus occurs in patients with chronic obstructive pulmonary disease and cystic fibrosis. Key steps in the mucus transport process are airway epithelia sensing and responding to changes in mucus hydration. We reported that extracellular adenosine triphosphate (ATP) and adenosine were important luminal autocrine and paracrine signals that regulated the hydration of the surface of human airway epithelial cultures through their action on apical membrane purinoceptors. Mucus hydration in human airway epithelial cultures was sensed by an interaction between cilia and the overlying mucus layer: Changes in mechanical strain, proportional to mucus hydration, regulated ATP release rates, adjusting fluid secretion to optimize mucus layer hydration. This system provided a feedback mechanism by which airways maintained mucus hydration in an optimum range for cilia propulsion. Understanding how airway epithelia can sense and respond to changes in mucus properties helps us to understand how the mucus clearance system protects the airways in health and how it fails in lung diseases such as cystic fibrosis.
Conflict of interest statement
Figures


References
-
- Paisley D, Gosling M, Danahay H. Regulation of airway mucosal hydration. Expert Rev Clin Pharmacol. 2010;3:361–369. - PubMed
-
- Xia B, Royall JA, Damera G, Sachdev GP, Cummings RD. Altered O-glycosylation and sulfation of airway mucins associated with cystic fibrosis. Glycobiology. 2005;15:747–775. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

